|
|
|
Indian Pediatr 2020;57:
762-763 |
 |
Transient Elastography to Represent Hepatic
Copper Accumulation in Wilson Disease
|
|
Jirakorn Jamrasnaradom1 and Palittiya
Sintusek2,3*
From 1Faculty of Medicine, 2Division
of Gastroenterology and Hepatology, Department of Pediatrics, King
Chulalongkorn Memorial Hospital; and 3Pediatric Liver
Diseases and Immunology STAR (Special Task Force for Activating
Research) and the Grants for Development of New Faculty Staff,
Ratchadaphiseksomphot Endowment Fund,
Department of Pediatrics; Chulalongkorn University,
Bangkok, Thailand.
Email: palittiya.s@chula.ac.th
|
|
Wilson disease is an autosomal recessive disorder
characterized by abnormal copper accumulation, diagnosed based on
clinical and laboratory features and treated with copper chelation
[1,2]. Recent studies show that transient elastography (TE) could be
used to predict liver fibrosis and monitor the disease progression [3];
however, many other conditions may lead to overestimation of the liver
stiffness. Herein, we report a large reduction of liver stiffness after
chelation therapy, that might be suggestive of effect of D-penicillamine
and zinc on reducing copper load.
A 13-year-old girl presenting with jaundice, poor
scholastic performance and coagulopathy for 6 months, was referred to
our hospital for liver transplantation. Physical examination revealed
Kayser-Fliescher (KF) rings with naked eye examination, splenomegaly,
and pedal edema. However, her neurological system was otherwise normal.
Laboratory investigations for Wilson disease and its complications were
performed including complete blood count (hemoglobin 11 g/dL, white
blood cell count 3,790/µL and platelet count 68,000/µL); liver function
tests (total bilirubin 3.8 mg/dL, direct bilirubin, 2.1 mg/dL, albumin
2.0 g/dL, globulin 3.6 g/dL, AST 85 units/L, ALT 56 units/L and ALP 419
units/L); serum copper 0.42 pm; zinc 0.296 pm; PT 31.5 sec, INR 2.68;
ceruloplasmin 9 mg/dL; and 24-hour urine copper 115.2 µg. Liver biopsy
was omitted due to uncorrectable coagulopathy. TE (FibroScan; Echosens,
Paris, France) was measured in preprandial state by a trained operator
which showed a value of 50.6 kilopascals (kPa).
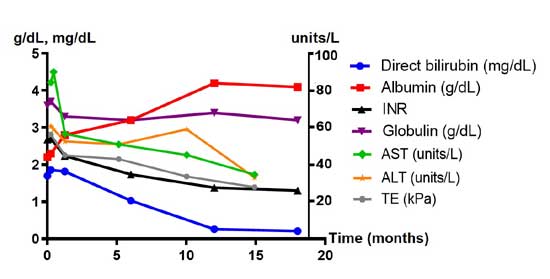 |
|
Fig. 1 Trend of laboratory parameters
and transient elastography (TE) in the index patient with Wilson
disease.
|
The patient was diagnosed with Wilson disease with 6
marks from the scoring system and the Wilson index score (WI) of 7,
which implies good outcome without liver transplantation. Hence, D-penicillamine
and zinc were initiated, and child closely followed-up for
deterioration, and worsening coagulopathy. Six weeks later, while her
clinical features and laboratory parameters did not improve, the TE
value dramatically decreased (36.8 kPa). After one year, she rejoined
school and performed well academically; her attention and memory were
improved as per feedback from parents and teachers. However, KF rings
were still present, even though tests of liver function were normal. The
TE value was 34.3, 22.8 and 15.7 kPa at 6, 12 and 18 months,
respectively, after the chelation therapy.
TE was used to measure liver stiffness by using a
share wave method which determines the fibrosis level. TE has been
studied as a non-invasive parameter to assess change in hepatic fibrosis
during treatment in patients with Wilson disease; the cut-off values of
mild and significant hepatic fibrosis were 6.6 and 8.4 kPa, respectively
[3,4]. The decreasing value after copper chelation was ascribed to
reduction in hepatic fibrosis [4]. However, liver elasticity may be
influenced not only by fibrosis but also by other factors such as liver
inflammation, the accumulation of various materials in liver tissue
[4,5] and liver congestion [6]. Mikund, et al. [6] found a rapid
decrease of liver stiffness from 73 kPa to 31 kPa in patients diagnosed
with Budd Chiari syndrome after endovascular procedure that suggested
the usefulness of TE in assessing hepatic congestion. Stefanescu, et
al. [4] also reported reduction of liver stiffness after one year of
treatment in children diagnosed Wilson disease. This study implied that
intrahepatic copper deposit might be involved in the high liver
stiffness before chelation therapy was initiated [4]. The present case
demonstrated the reduction of liver stiffness after chelation therapy,
with values comparable with the previous studies [4-6].
Consequently, the very high value of TE in the
present case might reflect not only fibrosis but also the copper
accumulation and inflammation in liver. Unfortunately, we could not
measure the liver copper content as the patient had uncorrectable
coagulopathy at the time of presentation. However, after chelation
therapy, the TE value steadily decreased, which was associated with an
improving clinical status. This report suggests the possibility of using
TE to represent hepatic copper accumulation and to monitor treatment of
Wilson disease.
REFERENCES
1. Ferenci P, Caca K, Loudianos G, Mieli-Vergani G,
Tanner S, Sternleib I, et al. Diagnosis and phenotypic
classification of Wilson disease. Liver Int. 2003;23:139-42.
2. Dhawan A, Taylor RM, Cheeseman P, De Silva P,
Katsiyiannakis L, Mieli-Vergani G. Wilson’s disease in children: 37-year
experience and revised King’s score for liver transplantation. Liver
Transpl. 2005;11:441-8.
3. Sini, M., Sorbello O, Civolani A, Liggi M, Demelia
L. Non-invasive assessment of hepatic fibrosis in a series of patients
with Wilson’s Disease. Dig Liver Dis. 2012;44:487-91.
4. Stefanescu AC, Pop TL, Stefanescu H, Miu N.
Transient elastography of the liver in children with Wilson’s disease:
Preliminary results. J Clin Ultrasound. 2016;44:65-71.
5. Musallam KM, Motta I, Salvatori M, Fraguelli M,
Marcon A, Taher AT, et al. Longitudinal changes in serum ferritin
levels correlate with measures of hepatic stiffness in
transfusion-independent patients with beta-thalassemia intermedia. Blood
Cells Mol Dis. 2012;49:136-9.
6. Mukund A, Pargewar SS, Desai SN, Rejesh S, Sarin
SK. Changes in liver congestion in patients with budd-chiari syndrome
following endovascular interventions: Assessment with transient
elastography. J Vasc Interv Radiol. 2017;28:683-87.
|
|
|
 |
|

