|
|
|
Indian Pediatr 2020;57: 723-729 |
 |
Guidelines on Diagnosis and Management of
Cow’s Milk Protein Allergy
|
|
John Matthai 1,
Malathi Sathiasekharan2,
Ujjal Poddar3,
Anupam Sibal4,
Anshu Srivastava3,
Yogesh Waikar5,
Rohan Malik6,
Gautam Ray7, S
Geetha8 and SK
Yachha9 for the
Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition;
Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics
From 1Masonic Medical Centre, Coimbatore, Tamil Nadu,
India; 2Kanchi Kamoti Child Trust Hospital, Chennai, Tamil
Nadu, India; 3Sanjay Gandhi Post Graduate Institute, Lucknow,
Uttar Pradesh, India; 4Apollo Hospital, New Delhi, India;
5Yogesh Waikar, Superspeciality GI Kids Clinics & Pediatric
Gastroenterology Unit, Nagpur, Maharashtra, India; 6All India
Institute of Medical Sciences, New Delhi, India; 7Institute
of Post Graduate Medical Education and Research, Kolkata, West Bengal,
India; 8Aster Medicity, Cochin, Kerala, India; and 9Sakra
World Hospital, Bangalore, Karnataka, India.
Correspondence to: Prof John Matthai, Pediatric Gastroenterologist,
Masonic Medical center, Race course, Coimbatore 641004. Email:
[email protected]
|
|
Justification: Cow’s
milk protein allergy (CMPA) is increasingly being diagnosed
in the West, while there is scant data on the subject from
India. There is low awareness among pediatricians about its
diagnosis and management; leading to improper diagnosis.
Process: A group of experts from the pediatric
gastroenterology sub-specialty chapter of Indian Academy of
Pediatrics (Indian Society of Pediatric Gastroenterology,
Hepatology and Nutrition) met at Mumbai on 26 October, 2018
and discussed various issues relating to the subject. A
broad consensus was reached and a writing committee was
formed. They met again on 11 August, 2019 at Chennai for a
detailed discussion. The statement was sent to the entire
group by e-mail and their approval obtained. Objective:
To formulate a consensus statement enable proper diagnosis
and management of Cow’s milk protein allergy.
Recommendations: Cow’s milk protein allergy is most
common in the first year of life. Gastrointestinal
manifestations are usually non-IgE mediated and therefore
skin prick test and specific IgE levels are not useful in
diagnosis. Clinical response to elimination diet followed by
a positive oral food challenge is diagnostic. In patients
with only gastrointestinal manifestations, sigmoidoscopy and
rectal biopsy may be considered as an alternative.
Management involves strict avoidance of all forms of bovine
milk protein. For infants who are artificially fed, an
extensively hydrolyzed formula is the first choice. Soy
formula is an alternative in those above six months of age.
Since most infants outgrow the allergy, elimination diet is
only for a limited period and re-evaluation should be done
periodically.
Keywords: Extensively hydrolyzed
formula, Food allergy, Non-IgE mediated, Oral food
challenge, Rectal biopsy.
|
|
F
ood allergy is an adverse immunological response
to proteins in food and must be differentiated from food intolerance,
which is a general non-specific term for any adverse reactions to
particular constituents of food. Cow’s milk protein allergy (CMPA) is an
immune- mediated reaction to various proteins in cow’s milk. It is the
most common food protein allergy in infants and children [1]. The
reaction may be IgE-mediated, non-IgE mediated or mixed. CMPA may have
cutaneous, respiratory and/or gastrointestinal manifestations.
In India, awareness among pediatricians is low
leading to misdiagnosis or concurrence with parents that the child has
allergy. This results in wrong dietary advice and unnecessary use of
expensive formulas. The prevalence of CMPA peaks in infancy (1.5 -3 %)
and falls to less than 1% at 6 years of age [2]. About 10 to 15% of
children who have CMPA are also allergic to soy and the risk of cross-
allergy is higher if symptoms begin below 6 months of age [3]. There are
no epidemiologic studies on the prevalence of food allergy including
CMPA in Indian children. Among hospital-based studies, CMPA was reported
as a cause of malabsorption syndrome in 6% children of all ages and 13%
of children below 2 years with chronic diarrhea [4]. CMPA was the cause
in 35% children below 3 years of age presenting with chronic diarrhea in
another study [5].
PROCESS
The pediatric gastroenterology sub-specialty chapter
of Indian Academy of Pediatrics (Indian Society of Pediatric
Gastroenterology, Hepatology & Nutrition) organized a meeting of select
members of the group at Mumbai on 26 October, 2018. Brief presentations
on various aspects of the topic were followed by a detailed discussion.
A broad consensus was reached and a ten member writing committee was
formed. This committee met again on 11 August, 2019 at Chennai for a
detailed discussion. The statement was finalized and sent to the entire
group by e-mail. Suggestions were incorporated and consent was obtained
from all the members .
RECOMMENDATIONS
Diagnostic Modalities
A good reliable history and clinical examination are
the cornerstones of diagnosis. Common differential diagnosis like
infective colitis, celiac disease, gastro-esophageal reflux disease,
eosinophilic esophagitis, immune deficiency and persistent diarrhea
should be kept in mind. Empirical exclusion therapy without confirmation
of diagnosis is unscientific and best avoided.
Diagnostic Elimination Trial
Current European Society of Pediatric
Gastroenterology, Hepatology and Nutrition (ESPGHAN) practice guidelines
suggest that the initial diagnosis of CMPA should be made on the basis
of diagnostic elimination of cow’s milk proteins from the diet and then
it is to be confirmed by an oral challenge with CMP if there is a
response to the elimination diet [6]. Elimination should be total, and
particular attention should be paid to hidden sources of the antigen (e.g.
avoiding biscuits or cake). If symptoms do not improve with strict
elimination, the diagnosis of CMPA is unlikely.
Oral Food Challenge
A double blind placebo controlled food challenge is
the gold standard for diagnosing CMPA, though it has the disadvantages
of requiring a longer time to perform, needing patient and parents
co-operation and being expensive [6]. Hence, in most instances (except
in those with uncertain or questionable response to the initial oral
challenge), an open food challenge is done, wherein the child is
continued on a normal milk containing diet. If the patient remains
without symptoms for two weeks, then CMPA is ruled out. However, if
symptoms recur, then the diagnosis of CMPA is confirmed. Oral food
challenge (OFC) is the most specific test for diagnosing food allergy
and reliably distinguishes sensitization from clinical allergy. They are
more standardized in IgE- mediated reactions, and should be done under
medical supervision. However, in cases of severe anaphylaxis, the
patient should be on a therapeutic elimination diet straightaway [6].
This test is required before re- introduction of the allergen after
therapeutic elimination period is completed to confirm development of
tolerance.
Endoscopy and Biopsy
An Indian study
done on CMPA noted that sigmoidoscopy (82%) and rectal
biopsy (97%) gave best information in patients with gastrointestinal
manifestations of CMPA [7]. Histological changes are similar and
non-specific in all food allergies and therefore should be interpreted
only in the context of appropriate clinical setting. The most frequently
seen endoscopic findings are focal erythema, erosions and nodular
lymphoid hyperplasia in 40–90% of cases
[8]. The presence of more than 60 eosinophils in six high
power fields and/or more than 15–20 eosinophils per high power field is
highly suggestive for CMPA .
Other Methods
Scoring system for screening: The Cow’s
milk-related symptom score (CoMiSS) has most commonly been used [9].
However, there is no agreement on cut-off
values and it has poor sensitivity and specificity [10].
Until more studies are available from developing countries, CoMiSS
cannot be recommended as a screening tool in our setting.
Specific IgE antibodies to cow milk: Specific IgE
antibodies detect the presence of circulating antibodies against CMP.
However, positive IgE neither confirms allergy nor differentiates
between sensitization and clinical allergy [26]. Specific IgE tests are
not useful in the diagnosis of non-IgE mediated CMPA
.
Skin prick test: Skin prick tests are used to
detect the presence of IgE tissue bound antibodies. It can be considered
in IgE-mediated disease, but a positive test does not confirm allergy.
Wheal size of
³5 mm (³2
mm in an infant <2
year) is associated with a higher specificity. A negative skin test
rules out IgE-mediated reactions, with negative predictive values of
95%. The wheal size is significantly larger in children with persistent
disease compared to those who outgrow CMPA and therefore is useful as a
prognostic indicator [11]. Infants are generally less responsive to skin
prick tests. It is not validated in non-IgE mediated CMPA and may result
in false positive or false negative diagnosis [12].
Approach to Diagnosis
CMPA is a clinical diagnosis, and there is no single
test or biomarker that is pathognomonic of the condition. The ESPGHAN
criteria of 2012 have done away with routine intestinal biopsy [6].
Allergen elimination followed by oral food challenge, has been advocated
as the cornerstone of diagnosis.
A structured approach is needed for accurate
diagnosis and should start with an allergy focused history (including
family history) and physical examination. If the clinical features
discussed earlier are present in an infant or young child, CMPA should
be considered in the differential diagnosis. Clinical pointers that
suggest IgE-mediated disease are the involvement of two or more systems,
commonly the skin, gastrointestinal and respiratory tract. On the
contrary, non-IgE mediated disease (which is more common in India) may
manifest with only gastrointestinal symptoms. The expert group does not
advocate testing for cow’s milk protein (CMP) - specific IgE in serum as
a routine, considering the high cost and as it indicates only
sensitization (food elimination and challenge needs to be done for
diagnosis). However, It may be useful with acute/ life threatening
symptoms such as stridor, wheeze, angioedema and anaphylaxis. Here, the
food challenge may be delayed by a year if CMP- specific IgE is positive
and there is symptom resolution with an elimination diet. Approach to a
child with suspected CMPA is given in Fig.1.
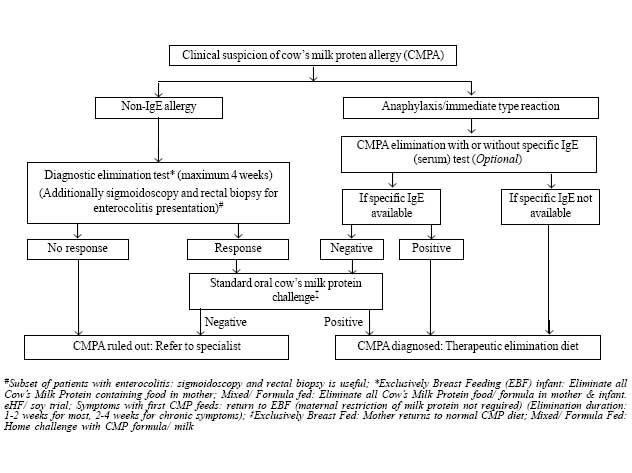 |
|
Fig. 1 Approach to a child with
suspected cow’s milk protein allergy.
|
The initial diagnosis of CMPA should be made on the
basis of a diagnostic elimination test. Response to CMP withdrawal is
noticed within 3-5 days for those with immediate manifestations, 1-2
weeks for those with delayed clinical manifestations, and 2-4 weeks for
those with chronic diarrhea/ failure to thrive [13]. If the child does
not show any improvement during this time period, a diagnosis of CMPA is
ruled out on most occasions. A few exceptions are: some children have
associated soy protein allergy or allergy to other components of the
extensively hydrolyzed formula (eHF) that has been used during milk
restriction; some sick infants may also have multiple food protein
allergies (such as egg, wheat, soy, nuts, sea fish). In both these
situations, an amino-acid based formulation (AAF) should be used during
the allergen elimination, and if there is no improvement in symptoms on
this too, then CMPA is ruled out as a cause for the child’s symptoms
[6].
For those on exclusive breastfeeding, elimination
requires excluding milk and milk products from the mother’s diet (while
she continues to breast feed the infant). Care must be taken to remove
sources of CMP from the breast-fed infants being given supplementary
feeds in addition. For non-breast fed patients, all sources of milk
protein should be stopped and infants should be started on an
extensively hydrolyzed formula. Soy formula may be used beyond 6 months
of age. For older children, all forms of milk and milk products should
be stopped as part of the elimination [6].
|
Box I
Clinical Manifestations of Cow’s Milk Protein Allergy |
|
IgE mediated syndromes (Onset- immediate to
<1 h)
Immediate food hypersensitivity, perioral
utricaria/erythema, angioedema/ anaphylaxisGeneralized rash,
vomiting, wheezing, cough
Non-IgE mediated (Onset - late >24 h, usually
after 5-7 d)
Proctocolitis: Fresh bleeding per rectum,
constipation
Enteropathy: Watery diarrhea, failure to thrive,
protein losing enteropathy, occult gastrointestinal bleeding
Enterocolitis: Bloody diarrhea, anemia/hypo-proteinemia
Esophagitis: Reflux like symptoms, vomiting/feed
refusal, dysphagia
Gastritis/Gastro-duodenitis: hematemesis, occult
gastrointestinal bleed
Atopic dermatitis
Mixed (Onset-intermediate, <24 h)
Food Protein Induced Enterocolitis syndrome
(FPIES): Vomiting/diarrhea/colitis, shock like symptoms with severe
vomiting, diarrhea, neutrophilic leukocytosis and metabolic acidosis
|
Patients who show an improvement of symptoms with
allergen elimination (as above) should be subjected to an oral milk
challenge after 2-4 weeks of a CMP free diet in the asymptomatic period
to confirm the diagnosis [13].
Procedure for oral food challenge: CMP either as
formula or pasteurized milk (in <12 months age) or pasteurized milk ( in
>12 months age) is administered cautiously in the following manner: 1 mL,
3 mL, 10 mL, 30 mL, 100 mL (given every 30 minutes), which can be done
on an out-patient basis [14]. The child should be observed for two
hours, and then sent home with an instruction to continue at least 200
mL of milk/day and to stop if there is recurrence of symptoms. The child
should be reviewed after two weeks to decide whether to continue milk or
to stop milk again depending on the clinical response to milk
introduction. For those with severe reactions on initial presentation (IgE-type),
the milk challenge is administered in an even more graded fashion (0.1
mL, 0.3 mL, 1 mL, 3 mL, 10 mL, 30 mL, 100 mL: given every 30 minutes) as
an in-patient with all resuscitation facilities including injection
adrenaline to manage anaphylaxis. A positive reaction to milk
introduction confirms the diagnosis of CMPA. If no reactions occur, 200
mL/day of milk is continued for two weeks to look for any delayed
manifestations.
Table I Differentiation Between Cow’s Milk Protein Allergy and Lactose Intolerance
|
Cow’s milk protein allergy |
Lactose intolerance |
|
Types |
IgE and Non-IgE mediated |
Congenital, primary (age-dependent decline in lactase enzyme)
and secondary (mucosal damage after severe gastroenteritis or
other causes) |
|
Mechanism |
All or none phenomenon, is an immune reaction to milk protein |
Quantity-dependent, due to deficient lactase enzyme in brush
border
|
|
Symptoms |
Multisystem (gastro-intestinal/skin/ respiratory |
Only gastrointestinal (diarrhea, flatulence, pain) |
|
Natural history |
Recovers by 4-5 y of age in majority |
Recovers in days-weeks in secondary, permanent in congenital and
primary |
Since non-IgE mediated gastrointestinal symptoms
appear to be the commonest manifestation of CMPA in India, sigmoidoscopy
and rectal biopsy can also be considered for confirmation of diagnosis
in children whose parents do not give consent for oral food challenge.
In children with no response to diagnostic elimination diet or those in
whom alternative diagnosis is strongly considered, further
investigations are necessary.
Management
The safest strategy for the management of CMPA is the
strict avoidance of CMP for a defined period [6]. A delay in diagnosis
may result in failure to thrive, anemia, and hypoproteinemia; however,
there is ample evidence that over-diagnosis or wrong diagnosis results
in unnecessary dietary restrictions, increased risk of rickets,
decreased bone mineralization and great economic burden [15]. The choice
of an appropriate substitute to fulfill the nutritional requirements
during the time of CMP avoidance is crucial. There are some variables
which should be considered before recommending alternatives to milk
feeds (Box II).
|
Box II
Factors to Consider When Deciding Alternatives to Bovine Milk |
|
Age: Whether older than or younger than 6 months
Feeding pattern: Exclusive breastfeeding,
mixed feeds (breastfeed and formula) or exclusive formula feeds.
Type of allergy: IgE-mediated or non-IgE
mediated.
Severity of reaction: Severe or mild to
moderate
Clinical manifestations: Gastrointestinal,
respiratory or skin.
Financial considerations: Affordability
|
Exclusively Breast-fed Infants
CMPA in an exclusive breast-fed infant is usually
mild and majority of these infants do not have anemia or failure to
thrive. Breastfeeding is continued till at least 6 months of age and the
mother is advised to avoid bovine milk and all dairy products (cheese,
yogurt, paneer, butter, ghee) as well as milk containing foods in her
diet. It may take up to 72 hours for the antigens to disappear from
breast milk and for clinical response after withdrawal of milk and milk
products [16]. The maternal elimination diet is maintained for 3 to 6
days in those with IgE-mediated allergy, while in non-IgE mediated it is
two weeks in those without atopy, and 4 weeks in those with atopic
dermatitis or allergic colitis [6]. If symptoms persist even after this
period, other allergens or a different etiology should be considered. If
the symptoms improve or disappear, CMP may be reintroduced as a
challenge in the maternal diet. If symptoms recur then CMP should be
avoided as long as she is breast-feeding. Calcium supplementation (1000
mg per day in divided doses) is essential for the mother during the
period of elimination. Very strict elimination diets that exclude not
only milk but also fish, soy, wheat and gluten products may cause
unnecessary nutritional imbalance in the mother and are best avoided.
Infants on Mixed Feeds
CMP is completely withdrawn along with all unmodified
animal (goat/sheep/buffalo/camel) milk proteins. However, breastfeeding
should be continued without any elimination in the maternal diet [6]. In
infants less than 6 months of age with mild to moderate reaction,
extensively hydrolyzed formula (eHF) with proven efficacy is recommended
[17]. There is safety concern regarding use of soy in infants less than
6 months of age and cross-allergy to soy is seen in in 10-15 % of
infants with CMPA. Soy is however cheaper and more palatable than eHF
and these factors should be weighed when alternate formula is
recommended. Children with IgE-mediated CMPA tolerate soy protein better
than non-IgE mediated CMPA. In infants more than 6 months of age with
mild to moderate reaction, soy protein formula can be used instead of
eHF if there are financial constraints [18]. If the diagnosis is
reasonably certain, but there is no improvement within 2 weeks of eHF,
then amino acid formula (AAF) should be tried before CMPA is ruled out.
In infants who are sick or have severe or life threatening symptoms AAF
should be the first choice rather than eHF [6].
Exclusive Formula Fed Infants
Breast feeds must be started if they were stopped
only recently. Rest of the management protocol is the same as in group
II.
A simple algorithm for management of infants with
non-IgE mediated CMPA is shown in Fig. 2.
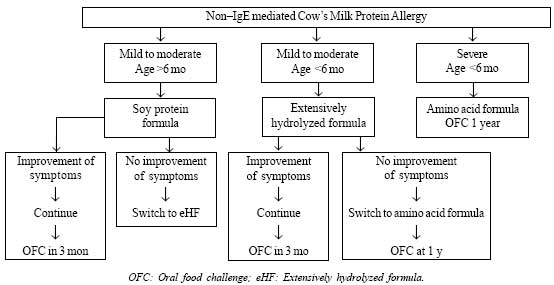 |
|
Fig. 2 Management of infants with non-IgE
mediated cow’s milk protein allergy.
|
Management of IgE-mediated CMPA
When an infant with CMPA presents with classical
symptoms of IgE-mediated allergy such as angioedema, urticaria or
anaphylaxis, emergency care should be provided as for any allergy, and
all forms of CMP should be immediately withdrawn. In mild to moderate
allergy, eHF is the first choice. Only those who do not respond to the
above measures should be switched to AAF. Those with severe allergy
require hospitalization and should be given only AAF. OFC should be done
with caution only in a hospital setting between 12 to 18 months. In
those with severe IgE mediated allergy, OFC should be done only with eHF
[16].
The elimination diet should be continued for atleast
one year and re-evaluation done every 6 months subsequently [40]. The
prognosis of infants and children with CMPA is good as 50% will tolerate
CMP by 1 year, >75% by 3 years and >90% by 6 years of age [19]. Only 5 %
would continue into adulthood. High total IgE and specific IgE levels
correlate with a higher age of acquiring tolerance [20].
Prevention
Primary prevention aims to delay the first exposure
of infants to cow’s milk protein, while secondary prevention involves
avoiding antigen exposure in high-risk atopic infants. Tertiary
prevention is when clinicians advise cow’s milk avoidance as a means of
treatment after confirmation of diagnosis.
Exclusively Breast-fed Infants
The best way to prevent CMPA is exclusive
breast-feeding for 4-6 months (17-27 weeks)[21]. The incidence of CMPA
is lower (0.5%) in exclusively breast-fed infants compared to
formula-fed or mixed-fed infants. The reproducible clinical reactions to
CMP are mild to moderate in the majority. A plausible explanation is
that the level of CMP in breast milk is 100, 000 times lower than that
in cow’s milk and is in the form of peptides and not as intact protein
[22]. Breast milk also contains proteases a digesting protein. The other
protective factors in breast milk are maternal antibodies and chemokines
which reduce the development of allergy, hormones and growth factors
which potentiate maturation of gut associated lymphoid tissue (GALT),
polyunsaturated fatty acids (PUFAs), glycoproteins, oligosaccharides and
micro RNAs which exhibit immune function.
There is no evidence that modification of maternal
diet during pregnancy or lactation has any protective effect against
allergy in at-risk infants. Moreover an exclusion diet may cause
nutritional deficiencies in the lactating mother and infant [23].
Allergen avoidance should be advised only when the breast-fed infant has
proven CMPA. There is no evidence to suggest that delaying introduction
of solid foods, or even potentially allergenic foods, beyond age 4-6
months offers any protective effect. Supplementary foods should be
introduced one at a time in small quantities, preferably while the
mother is still breastfeeding but not before the infant is at least 17
weeks of age to prevent other allergies [24].
Infants Not Exclusively Breast-fed
There is no role for milk formula with intact protein
from other animals or soy protein in the prevention of allergy. The role
of hydrolyzed formulae (both partial and extensively) in prevention of
CMPA is still debated. If there is a family history of allergic disease
in both parents, there may be some justification in using partially
hydrolyzed formula with whey protein as a starter formula, if exclusive
breastfeeding is not possible. However, a recent meta-analysis concluded
that there is no benefit of using hydrolyzed formula to prevent CMPA
[25].
CONCLUSION
CMPA is primarily a disease of infancy with
increasing incidence. While manifestations of IgE- mediated disease is
immediate with multi system involvement, non-IgE disease is delayed with
symptoms related to the GI tract. Clinical response to an elimination
diet followed by an oral food challenge is the cornerstone of diagnosis.
In children with persistent diarrhea and colitis, sigmoidoscopy and
biopsy are useful in diagnosis. Breastfeeding should be continued and
all cow’s milk protein should be stopped. Most children will outgrow the
allergy between 12 and 18 months of age.
Contributors: JM: coordinated and edited the
paper; SKY: verified the scientific content; ASr and RM: authored the
segment on prevalence and clinical features; AS and YW: the segment on
diagnostic modalities; UP,GR: the segment on approach to diagnosis,
MS,GS: the segment on management and prevention. All authors
participated in finalizing the paper.
Funding: None; Competing interests:
None stated.
| |
|
Recommendations
• In non-IgE mediated cows’ milk protein
allergy (CMPA), milk specific-IgE and skin prick tests are not
useful in diagnosis.
• Oral food challenge following a clinical
response to elimination diet is mandatory in diagnosis. In those
with only gastrointestinal manifestations, sigmoidoscopy and
rectal biopsy should be considered
• Duration of diagnostic milk protein
elimination needed to observe clinical response varies from 3-5
days in IgE-mediated allergy and 2-4 weeks in others.
• Unmodified mammalian milk (cow, buffalo,
donkey, goat or camel) should not be used in infants with proven
CMPA.
• In artificially-fed infants with CMPA,
extensively hydrolyzed formula is the first choice. Soy formula
may be considered above 6 months of age. Amino acid formulas are
needed only in a small subset of infants.
• Delaying introduction of solid foods, or
even potentially allergenic foods, beyond 4-6 months of age has
no protective effect in high risk infants.
|
REFERENCES
1. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer
L, Sodergren E, et al. The prevalence of food allergy: A
meta-analysis. J Allergy Clin Immunol. 2007;120:638-46.
2. Høst A, Halken S. A prospective study of cow milk
allergy in Danish infants during the first 3 years of life. Clinical
course in relation to clinical and immunological type of
hypersensitivity reaction. Allergy. 1990;45:587-96.
3. Klemola T, Vanto T, Juntunen-Backman K, Kalimo K,
Korpela R, Varjonen E. Allergy to soy formula and to extensively
hydrolyzed whey formula in infants with cow’s milk allergy: A
prospective, randomized study with a follow-up to the age of 2 years. J
Pediatr. 2002;140: 219-24.
4. Yachha SK, Misra S, Malik AK, Nagi B, Mehta S.
Spectrum of malabsorption syndrome in north indian children. Indian J
Gastroenterol. 1993;12:120-5.
5. Poddar U, Agarwal J, Yachha SK, Srivastava A.
Toddler’s diarrhea: Is it an under-recognized entity in developing
countries? J Trop Pediatr. 2013;59:470-5.
6. Koletzko S, Niggemann B, Arato A, Dias JA,
Heuschkel JJR, Husby S, et al. Diagnostic approach and
management of cow’s-milk protein allergy in infants and children:
ESPGHAN GI Committee Practical Guideline. J Ped Gastroententerol Nutr.
2012;55:221-9.
7. Poddar U, Yachha SK, Krishnani N, Srivastava A.
Cow’s milk protein allergy: An entity for recognition in developing
countries. J Gastroenterol Hepatol. 2010; 25:178-82.
8. Tataranu E, Diaconescu S, Ivanescu CG. Clinical,
immunological and pathological profile of infants suffering from cow’s
milk protein allergy. Rom J Morphol Embryol. 2016;57:1031-5.
9. Vandenplas Y; Althera Study Group, Steenhout P,
Grathwohl D. A pilot study on the application of a symptom-based score
for the diagnosis of cow’s milk protein allergy. SAGE Open Med. 2014;2:
2050312114523423.
10. Prasad R, Venkata RS, Ghokale P, Chakravarty P,
Anwar F. Cow’s milk-related symptom score as a predictive tool for cow’s
milk allergy in Indian children aged 0–24 months. Asia Pac Allergy.
2018;8:e36.
11. Petersen TH, Mortz CG, Bindslev-Jensenetal C.
Cow’s milk allergic children – Can component-resolved diagnostics
predict duration and severity? Pediatr Allergy Immunol. 2018;29:194-9.
12. Muraro A, Werfel T, Hoffmann Sommergruber
K, Roberts G, Beyer K, Bindslev Jensen C, et al. Food allergy and
anaphylaxis guidelines. Diagnosis and management of food
allergy. Allergy. 2014;69:1008-25.
13. Lozinsky AC, Meyer R, De Koker C, Dziubak R,
Godwin H, Reeve K, et al. Time to symptom improvement using
elimination diets in non-IgE mediated gastrointestinal food allergies.
Pediatr Allergy Immunol. 2015;2:317-29.
14. Niggemann B, Beyer K. Diagnosis of food allergy
in children: Towards a standardization of food challenge. J Pediatr
Gastroenterol Nutr. 2007;45:399-404.
15. Vandenplas Y, Abuabat A, Al-Hammadi S, Aly GS,
Miqdady MS, Shaaban SY, et al. Middle east consensus statement on
the prevention, diagnosis, and management of cow’s milk protein allergy.
Pediatr Gastroenterol Hepatol Nutr. 2014;17:61-73.
16. Lifschitz C, Szajewska H. Cow’s milk allergy:
Evidence-based diagnosis and management for the practitioner. Eur J
Pediatr. 2015;174:141.
17. Host A, Koletzko B, Dreborg S, Muraro A, Wahn U,
Aggett P et al. Dietary products used in infants for treatment
and prevention of food allergy. Joint statement of the European Society
for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on
Hypoallergenic Formulae and the European Society for Pediatric
Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on
nutrition. Arch Dis Child. 1999;81:80-4.
18. Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle
RJ, Cocco R, et al; wao special committee on food allergy and
nutrition. Clinical use of probiotics in pediatric allergy (CUPPA): A
World Allergy Organization position paper. World Allergy Organ J.
2012;5:148-67.
19. Høst A, Halken S, Jacobsen HP, Christensen AE,
Herskind AM, Plesner K. Clinical course of cow’s milk protein
allergy/intolerance and atopic diseases in childhood. Pediatr Allergy
Immunol. 2002;13:23-8.
20. Skripak JM, Matsui EC, Mudd K, Wood RA. The
natural history of IgE-mediated cow’s milk allergy. J Allergy Clin
Immunol. 2007;120:1172-7.
21. Friedman NJ, Zeiger RS. The role of
breast-feeding in the development of allergies and asthma. J Allergy
Clin Immunol. 2005;115:1238-48.
22. Vandenplas Y. Prevention and management of cow’s
milk allergy in non-exclusively breastfed infants. Nutrients. 2017;9:
731;1-15.
23. Kramer MS, Kakuma R. Maternal dietary antigen
avoidance during pregnancy or lactation, or both, for preventing or
treating atopic disease in the child. Cochrane Database Syst Rev.
2006;3: CD000133.
24. Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek
S, Koletzko B, et al. Complementary feeding: a commentary by the
ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;
46:99-110.
25. Osborn DA, Sinn JK, Jones LJ. Infant formulas containing
hydrolyzed protein for prevention of allergic disease. Cochrane Database
Syst Rev. 2018;10: CD003664.
|
|
|
 |
|

