|
|
|
Indian Pediatr 2019;56: 673-681 |
 |
Infantile Thiamine Deficiency: New Insights
into an Old Disease
|
|
Mudasir Nazir 1,
Roumissa Lone2
and Bashir Ahmad Charoo3
From Departments of Pediatrics; 1Shri Mata
Vaishno Devi Narayana Hospital, Kakryal; 2Government Medical
College Jammu, and 3Sher-I-Kashmir Institute of Medical
Sciences Hospital, Srinagar; Jammu & Kashmir, India.
Correspondence to: Dr Mudasir Nazir, Department of
Pediatrics and Neonatology, Shri Mata Vaishno Devi Narayana Hospital,
Kakryal, Jammu, Jammu & Kashmir 182 320, India.
Email: [email protected]
|
|
Context: The wide spectrum of
clinical presentation in infantile thiamine deficiency is difficult to
recognize, and the diagnosis is frequently missed due to the lack of
widespread awareness, and non-availability of costly and technically
demanding investigations. Evidence acquisition: The topic was
searched by two independent researchers using online databases of Google
scholar and PubMed. We considered the related studies published in the
last 20 years. The terms used for the search were ‘thiamine’, ‘thiamine
deficiency’, ‘beri-beri’, ‘B-vitamins’,‘micronutrients’, ‘malnutrition’,
‘infant mortality’. ‘Wernicke’s syndrome’,‘Wernicke’s encephalopathy’,
and ‘lactic acidosis’. Results: In the absence of specific
diagnostic tests, a low threshold for a therapeutic thiamine challenge
is currently the best approach to diagnose infantile thiamine deficiency
in severe acute conditions. The practical approach is to consider
thiamine injection as a complementary resuscitation tool in infants with
severe acute conditions; more so in presence of underlying risk factors,
clinically evident malnutrition or where a dextrose-based fluid is used
for resuscitation. Further, as persistent subclinical thiamine
deficiency during infancy can have long-term neuro-developmental
effects, reasonable strategy is to treat pregnant women suspected of
having the deficiency, and to supplement thiamine in both mother and the
baby during breastfeeding. Conclusions: Health care professionals
in the country need to be sensitized to adopt a high level of clinical
suspicion for thiamine deficiency and a low threshold for the
administration of thiamine, particularly when infantile thiamine
deficiency is suspected.
Keywords: Beri-beri, Micronutrients,
Mortality, Nutrition, Vitamin B.
|
|
T
hiamine is a water-soluble B vitamin that plays
important co-enzymatic and non-co-enzymatic roles within the body [1].
In addition to its role in the metabolism of carbohydrates and
amino-acids, thiamine is essential in the synthesis of nucleic acids,
myelin, and neurotransmitters (acetylcholine) [1]. Recent evidence
suggests that thiamine may have a role in immunity, anti-inflammation
and gene regulation [1-2]. Thiamine is an essential vitamin with no
endogenous source of synthesis within humans and needs to be
continuously supplied in the diet. In addition, the body stores are
limited and the turnover rate is high (half-life <10 days) making it
potentially susceptible to depletion. In conditions of insufficient
intake, thiamine deficiency can develop over a period of 2-3 months
[3,4].
The global prevalence of thiamine deficiency is
poorly documented due to a dearth of population-level biomarker data
[5]. Studies from South-East Asia have reported a prevalence of 27-78%
in mothers and 15-58% in children [1,3,5] . The prevalence in children
admitted to hospitals ranges from 13-30% in South Asia and around 40% in
Africa [3,5,6]. In India, there are limited reports of thiamine
deficiency in the pediatric population [7-9].
In infancy, thiamine deficiency has a wide range of
clinical presentations, with high fatality in untreated cases, and
survivors usually have long-term sequelae. Although thiamine deficiency
is effectively treatable, it continues to affect infants in both
developed and underdeveloped countries, and with potentially serious and
life-threatening consequences [3,8-10]. This review was undertaken in
view of recent reports of infantile thiamine deficiency from this region
in Northern India. [13-18]. The review also becomes important as current
research suggests role of thiamine deficiency in sepsis/septic shock,
and induced-thiamine deficiency in re-feeding syndrome [3]. This review
was further prompted by longitudinal evidence suggesting potential
adverse long term implications of subclinical infantile thiamine
deficiency on neuro-development in later childhood [19-21].
Thiamine Biology
Thiamine (vitamin-B1) is a water-soluble vitamin
found in several food products including meat, fish, seeds, nuts, green
peas, sunflower seeds, beans, and soy products [5,22]. In children, the
estimated daily recommended dietary allowance (RDA) is 0.5mg/day for 1-3
years, 0.6 mg/day for 4-8 years, 0.9 mg/day for 9-13 years, and 1-1.2
mg/day for 14-18 years of age [22]. The RDA for adult men is 1.1 mg/day,
adult women is 1.2 mg/day; and for women during pregnancy and lactation
RDA is 1.4 mg/day [22]. Breast milk has a thiamine content of around
0.21 mg/L but it may vary depending on the diet and the geographical
region [5,22].
Absorption: Thiamine absorption is most efficient
in the upper jejunum and to a lesser amount in the duodenum and ileum
[23]. Thiamine is absorbed in its free, non-phosphorylated form into the
intestinal mucosal cells [23,24]. The small intestine has a dual system
of thiamine absorption either through an active carrier-mediated or
via a passive diffusion process [23-25]. Once inside the mucosal
cell, thiamine is phosphorylated to thiamine diphosphate by thiamine
pyrophosphokinase, before it is transported to the opposite pole [25].
Distribution: On the basolateral membrane of
intestines, thiamine is transported by a thiamine/H+ antiport system
into the portal circulation [26]. Thiamine targets the cells that
utilize glucose as the main energy source; however, thiamine tissue
tropism is primarily determined by the degree of expression of key
transporters on cell membranes in the major body systems of splanchnic,
muscular, nervous, renal systems, and the placenta [27, 28].
Pathophysiology of Deficiency
Thiamine is present in the body as free thiamine, as
well as in several phosphorylated forms: thiamine mono-phosphate (TMP),
thiamine diphosphate (TDP), and thiamine triphosphate (TTP). TDP also
called thiamine pyrophosphate, is the metabolically active and the most
abundant form of thiamine in the body (>80%) [29,30]. Thiamine plays
essential coenzyme and non-coenzyme roles in energy transformation,
synthesis of pentoses and nicotinamide adenine dinucleotide
phosphate(NADPH), and membrane and nerve conduction [29]. In energy
transformation, thiamine is a cofactor in multiple enzyme complexes
involved in the metabolism of carbohydrates and amino acids,
particularly pyruvate dehydrogenase complex (PDH), and
a-ketoglutarate
dehydrogenase complex (a–KGDH)
[31] (Fig. 1).
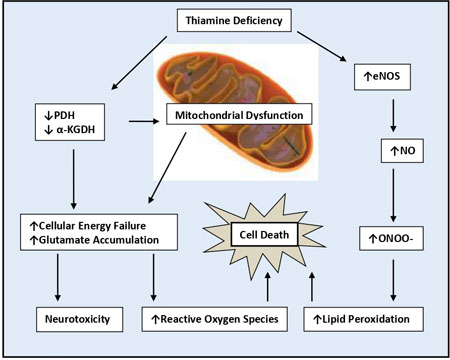 |
|
Fig. 1 Thiamin deficiency induced
neurotoxicity, lipid peroxidation, and cell death.
a- KGDH-a-ketoglutarate
dehydrogenase; eNOS-epithelial nitric oxide synthase; NO-nitric
oxide; ONOO–peroxynitrate; PDH-pyruvate dehydrogenase.
|
There are fundamental variations in the distribution
of thiamine derivatives in human brain, with compart-mentalization of
thiamine dependent enzymes in areas specifically involved in cerebral
glucose and energy utilization [1-3]. Therefore thiamine deficiency
causes preferential injury in areas which have high metabolic
requirement and high thiamine turnover rate [3]. This explains the
specific brain imaging findings with dominant involvement of basal
ganglia, which are known to have abundant mitochondrial density and a
rich vascular supply [15,17]. Further, studies have reported that
transketolase present in myelinated neurons is responsible for
maintaining myelin sheaths. The neurological aberrations observed in
thiamine deficiency may, therefore, be due to a lack of energy, a
decreased amount of acetylcholine, and/or a reduction in nerve impulse
transmission [1,32]. Similarly, muscle cells, particularly cardiac
myocytes, with high energy utilization are predominantly involved in
thiamine deficiency, giving rise to early manifestations such as the
muscle weakness, paresis of gastrointestinal tract, pulmonary
hypertension and heart failure [3,13].
Risk Factors for Thiamine Deficiency
Thiamine deficiency is rare in healthy individuals in
food-secure settings, where access to thiamine-rich foods ensures
adequate intakes. Deficiency can result from various mechanisms which
include: decreased nutrient intake, increased nutrient losses, impaired
nutrient absorption or increased demand [3,33,34] (Box I).
|
Box I Risk Factors for Thiamine
Deficiency Disorders
Decreased nutrient intake
• Low socioeconomic status
• Rural background
• Monotonous diets based on
milled white cereals, like polished rice (the rich thiamine
envelop removed by polishing and repetitive washing) and wheat
flour
• Customary dietry
restriction
• Exclusive breast feeding
• Delayed introduction of
complementary feeding
• Starvation
• Patients on Total
parenteral nutrition
• Anti-thiamine factors in
diet like tea leaves, betel nuts, coffee, fermented raw fish,
mycotoxins
Increased nutrient losses
• Renal loss – loop
diuretics, osmotic diabetic dieresis
• Digestive losses – chronic
diarrhea, hyperemesis
• Hemodialysis, continuous
renal replacement therapy
Increased demand
• Pregnancy
• Lactation
• Critical illness
• Refeeding syndrome
• High carbohydrate or
saturated fat diets
• Heavy alcohol drinking
• Inadequate thiamine-caloric
ratio in dextrose-based fluid resuscitation
• Vaccination
Impaired absorption
• Impaired intestinal
absorptive capacity during malnutrition
• Tropical enteropathy
• Secondary to surgical resection of large
portions of the gastrointestinal tract
|
Risk factors in infancy: Infants are particularly
susceptible to thiamine deficiency in the initial months of life, and
exclusively breastfed infants of thiamine-deficient but otherwise
asymptomatic mothers are at the highest risk. Studies have shown that
thiamine content in breast milk is directly related to the status of
thiamine in the nursing mother [34,35]. Additionally, certain customary
habits like dietary restrictions in mothers also contribute to the
deficiency in some communities. Further, associated co-morbidities are
common in infants and increase the risk of thiamine deficiency, like
sepsis and shock are frequent during complicated severe acute
malnutrition and contribute to the increased mortality [5]. In developed
countries, infantile thiamine deficiency outbreaks have been
periodically described [10,36]. One such outbreak in Israel in the year
2003 was due to thiamine-deficient soya formula, and had a high fatality
rate [10]. Infantile thiamine deficiency is sometimes reported in
intensive care units in patients receiving total parenteral nutrition
without thiamine supplementation or in patients receiving prolonged but
inadequate thiamine dose [36]. Recently, thiamine deficiency is
increasingly being recognized in infants with delayed introduction of
complementary diet in at-risk populations [5].
The recent reports of thiamine deficiency from
Kashmir were mainly attributed to the local diet that largely consists
of polished, unfortified rice [13-18]. All the cases occurred in infants
who were exclusively breastfed, and most mothers followed a customary
dietary restriction during the postpartum period.
Spectrum of Clinical Presentation
Thiamine deficiency classically known as beriberi has
a wide range of clinical presentation in infants. Based on the age three
clinical forms have been identified in infants: pernicious or cardiac,
aphonic form, and pseudo-meningitic form [4,37] (Box II). Whilist,
the dominant organ system involvement varies considerably in different
Indian studies, there is a consistent pattern in terms of underlying
risk factors for thiamine deficiency [7,8,13,14] (Table
I).
|
Box II Clinical Spectrum of Thiamine
Deficiency Disorders
Pernicious or acute cardiac
form
• Peaks at 1- 3 mo of age,
starts with non-specific symptoms
• Refusal to feed
• Emesis, constipation
• Tachypnea
• Agitation
• Loud piercing incessant
crying progressing to aphonia.
• Acute congestive cardiac
failure with cyanosis and edema.
• Rapidly progressive and
fulminant form with no edema (Shoshin beriberi) in certain
infants.
Aphonic form
• Less severe form:
predominates at 4–7 mo
• Aphonia due to paresis (or
paralysis) of the vocal cords
• Untreated cases advance
into cardiac and respiratory failure, death within days-weeks.
Pseudomeningitic form: 6-12 mo old.
• Muscular fasciculation
• Nystagmus, Ophthalmoplegia
• Tense fontanel
• Seizures, and coma
• Clinical signs of
meningitis, but cerebrospinal fluid findings excludes infection.
Encephalopathic form
• Usually older children and
adults, sometimes in infants
• Ophthalmoplegia, nystagmus
• Ataxia.
• Reduced consciousness
• Coma and death.
• A truncated Wernicke-like
syndrome with-out ataxia may also develop in some children
Neuropathic form
• Latter half of infancy,
older children and adults
• Muscle pains
• Diminished or abolished
deep tendon reflexes
• Ataxia
• Muscle wasting
• Cranial nerve involvement
|
TABLE I Clinical Characteristics of Thiamine Deficiency Reported in Different Indian Studies
|
Bhat, et al. |
Qureshi, et al. |
Rao, et al. |
Rao, et al. |
|
2017 [11] |
2016 [12] |
2010 [36] |
2008 [35] |
|
Study sample size (n) |
29 |
23 |
55 |
166 |
|
Age at presentation (mo) |
2.6 |
1.7 |
3.9 |
7 |
|
Exclusive breast-fed, % |
100 |
100 |
100 |
100 |
|
Dominant clinical syndrome |
PAH |
Life-threatening metabolic |
PAH with right |
Infantile
|
|
|
acidosis |
heart failure |
encephalitic |
|
Systemic features, % |
|
Fever |
31 |
21 |
52.7 |
72.2 |
|
Reduced feeding |
– |
34 |
– |
– |
|
Failure to thrive |
– |
4 |
– |
– |
|
Reflux
|
– |
56 |
– |
44 |
|
Cardiovascular, % |
|
Tachycardia |
86.2 |
100 |
100 |
– |
|
Poor perfusion |
75.8 |
52 |
– |
– |
|
Edema |
65.5 |
– |
10.9 |
– |
|
TR murmur |
93 |
– |
– |
– |
|
Respiratory, % |
|
Tachypnea |
68.9 |
– |
100 |
– |
|
Gasping breathing |
17.2 |
– |
– |
93.5 |
|
Apnea
|
– |
- |
– |
6.5 |
|
Hoarsenes of voice and/or aphonia |
– |
4 |
– |
18.2 |
|
Central nervous system, %
|
|
Irritability |
82.7 |
82 |
– |
– |
|
Lethargy |
– |
8 |
– |
63.3 |
|
Vacant stare |
13.7 |
13 |
– |
– |
|
Ptosis |
– |
13 |
7.3 |
76 |
|
Seizures |
– |
26 |
– |
55.4 |
|
Moaning
|
– |
73 |
– |
– |
|
Gastrointestinal, %
|
|
Diarrhea |
_
|
13 |
– |
– |
|
Hepatomegaly |
100 |
– |
– |
80 |
|
PAH: pulmonary arterial hypertension; TR: tricuspid
regurgitation. |
Long-term and Subclinical Consequences
Infants who survive the severe acute thiamine
deficiency may demonstrate marked intellectual and motor disabilities,
microcephaly, seizures, auditory impairment, and various degrees of
heart block [19]. In addition to the acute clinical forms described,
more subtle and predomi-nant neurological impairments have also been
reported and ascribed to underlying chronic subclinical thiamine
deficiency in infancy. These include abnor-malities in cognitive and
psycho-motor development, aberrations in syntactic and lexical
modalities of language acquirement, and seizures [20,21]. Longi-tudinal
studies of the survivors of 2003 Israeli outbreak of thiamine deficiency
have reported long-term neuro-logical, developmental, and gross motor
impairments in children with persistent subclinical deficiency in the
first year of life [10,20,21].
Severe Acute Clinical Scenarios Associated with
Thiamine Deficiency
Common differentials for thiamine deficiency in
infants include sepsis, encephalitis, meningitis, cardiomyopathy,
seizure disorder, cerebral malaria, infantile kwashiorkor, vitamin A
intoxication, Leighs disease, metabolic encephalopathy, idiopathic
pulmonary arterial hyper-tension, among others [37].
Functional or true thiamine deficiency has been found
to be associated with various severe acute conditions in children and
adults. In a Brazilian study, the prevalence of thiamine deficiency was
28% in sick infants admitted to pediatric intensive care units [38], and
there was documented biochemical evidence of deficiency in approximately
13.4% of critically ill infants without actual clinical evidence of
beriberi [40]. This may be a reason for the poorer prognosis of septic
shock in complicated severe acute malnutrition, with potential thiamine
deficiency precipitated by sepsis [39]. Moreover, the development of
re-feeding syndrome during the management of severe acute malnutrition
(SAM) may contribute to the higher mortality, particularly when there is
rapid introduction of feeds in children with pre-existing depleted body
stores of thiamine. During nutritional resuscitation, rapid commencement
of feeds triggers insulin production leading to enhanced protein
synthesis and heightened cellular glucose metabolism, and consequent
higher metabolic thiamine utilization and demand [41-43]. This induced
deficiency along with the signs of re-feeding syndrome are often
over-looked or misinterpreted as sepsis, pneumonia, encephalitis,
cardiac failure or sudden death [42].
In addition, recent studies have attributed
underlying thiamine deficiency for the increased mortality in patients
with lactic acidosis in acute severe conditions and shock [39,44].
Moreover, in intensive care units, risk of deficiency increases during
hospital stay, as sick children are often fasting for prolonged periods,
and parenteral nutrition is most often lacking, more so in resource-poor
settings.
Broadly, in the acute care setting, underlying
thiamine deficiency should be suspected in children with persistent
lactic metabolic acidosis or elevated plasma anion-gap, cardiogenic
shock unresponsive to appropriate therapy, and in any condition that
results in increased thiamine demand (hypermetabolic states) such as
sepsis, shock, poly-trauma, large burns, diabetic ketoacidosis,
congenital heart disease, and severe malaria [3-5,14,32,38]. Further,
thiamine deficiency should be kept as a possibility whenever there are
unexplained severe neurological signs in infants without clinical
evidence of true thiamine deficiency [3].
Evaluation
Thiamine status can be determined by analysis of
plasma, serum or whole blood; however, it represents only a small part
of the whole body thiamine pool [11]. TDP levels provide a better
measure of body thiamine status but do not assess thiamine metabolic
function. Erythrocyte transketolase activity (ETKA) is more accurate in
assessing the functional thiamine status of the body (Table II).
Thiamine is excreted in urine, mainly as free thiamine and TMP, and
levels <40 µg/day or <27 µg/g creatinine can be taken as suggestive of
thiamine deficiency [3,4,11].
TABLE II Biomarkers Used to Measure Thiamine Status
|
Biomarker |
Specimen |
Normal value |
Advantages |
Disadvantages |
|
Direct assessment
|
|
Thiamine
|
Plasma |
75 to 195 nmol/L |
Indicates recent intake |
Represents a small
|
|
|
|
|
part (<10%) of the whole body
|
|
|
|
|
thiamine pool
|
|
|
|
|
Low specificity and sensitivity |
|
ThMP |
Plasma
|
|
Indicates recent intake |
Not an indicator of thiamine
|
|
|
|
|
status |
|
ThDP |
Whole blood |
70 to 180 nmol/L |
Dominant form (~80%) |
Does not assess thiamine
|
|
Erythrocytes
|
|
of thiamine in erythrocytes. |
metabolic function. |
|
|
|
Better measure than total |
Unstable if specimen is not
|
|
|
|
thiamine. |
handled properly. |
|
Indirect/functional assessment |
|
ETKA |
Washed erythrocytes. |
Increase of >25% |
Functional assay of
|
Expensive
|
|
Increase in ETKA |
indicates high risk of |
biological activity |
Time consuming
|
|
with the addition of |
deficiency,
|
|
Not readily available |
|
thiamine to the
|
Increase between
|
|
|
|
incubation medium |
16% and 25% indicates
|
|
|
|
|
moderate risk |
|
|
|
ThMP: thiamine monophosphate; ThDP: thiamine diphosphate;
ETKA: erythrocyte transketolase activity. |
Concentration of pyruvate or lactate in the blood can
also be used to assess the thiamine status but these measurements are
limited by a lack of specificity [11,14]. Specific lesions in certain
areas of the brain on MR imaging can be helpful in early identification
of neurologic involvement in thiamine deficiency. MRI of Wernicke’s
syndrome in infants displays lesions in the frontal lobe and basal
ganglia, chiefly the striatum and putamen. In addition, both adults and
children with thiamine deficiency exhibit the same symmetrical
high-intensity signal on T2 weighted MRI in mammillary bodies,
peri-aqueductal and thalamic areas [7,15,17]. MR findings reported in
Western literature also demonstrated lesions in the basal ganglia and
frontal lobes [45]. However, Indian studies reported dominant basal
ganglia (putamina) lesions with infrequent involvement of thalamic,
cortical, brainstem and mamillary bodies [15,35]. Recently, cranial
ultrasono-graphy was observed to have utility as a first-line screening
and diagnostic tool in infantile encephalitic beri-beri [15]. Basal
ganglia hyperechogencity on neurosonogram was reported to have a
sensitivity and specificity of 71% and 92%, respectively, with maximum
sensitivity in Wernicke-like syndrome at 90% and least in the acidotic
form at 43% [15].
Treatment
Though thiamine assessment prior to repletion may be
used to confirm the suspected deficiency, serious and potentially
irreversible neurologic damage can occur in untreated cases. In such
contexts the ideal approach is a high index of clinical suspicion and
early therapeutic thiamine challenge, which is the treatment of
suspected cases without laboratory confirmation and monitoring for the
resolution of signs and symptoms [36]. Considering the safety profile
and a wide dosage range (50 to 1500 mg) in such cases, thiamine can be
administered as a slow intravenous injection. In severe acute conditions
due to thiamine deficiency, rapid clinical improvement occurs (within
hours or days) following thiamine administration, with neurological
involvement requiring higher doses and often taking a longer time to
recover (few days) [4,11]. Treatment or prevention of induced-deficiency
in refeeding syndrome needs proper adjustments in volume and calorie
density of feeds, gradual correction of electrolyte disturbances and
adequate supplementation of thiamine in therapeutic diets. Current
recommendation is to administer 2 mg/kg of thiamine daily during the
first week of SAM management [46,47]. As ready-to-use therapeutic foods
(RUTF) [either F-75 (75 kcal/100 mL) or F-100 (100 kcal/100 mL)] contain
an average of 0.5 mg of thiamine per sachet, proper attention to
additional supplementation is needed during the initiation phase of SAM
management [48]. Moreover, infants under 6 months of age with SAM
receive either breast milk or diluted RUTF, putting them at higher risk
of thiamine deficiency, particularly when the mothers are not properly
supplemented. Therefore, Infants under 6 months need to be supplemented
with 2 mg/kg of thiamine daily in order to mitigate the risk of inducing
thiamine deficiency during SAM management [3,5,46,47].
Current Indian Scenario
Most of the literature on micronutrients relating to
the Indian scenario focuses on deficiencies of iron, vitamin A and
iodine, and less attention has been given to vitamin B deficiencies,
including thiamine. The actual prevalence and potential contribution of
thiamine deficiency disorders to the infant mortality in India are not
known and is mostly considered as an association with other deficiencies
in severe acute malnutrition [9].
Though most of the studies on infantile thiamine
deficiency are from South Asian countries, it has been reported from
different parts of India as well. One study from India reported a high
prevalence of a form of infantile encephalitis with overlapping features
of Leigh’s disease, with a dramatic response to thiamine
supplementation, suggesting a diagnosis of thiamine deficiency. The
diagnosis was later confirmed in most of the patients by ETKA analysis
[7]. This study highlighted the importance of thiamine deficiency in
Indian context after it was reported to have been eliminated from India
in 2004 [49]. A review on micronutrient deficiencies in Indian children
concluded that sub-clinical B vitamin deficiencies are quite rampant in
India, and that they are likely to have long-term functional effects
that track into adulthood [48]. More recently, the reports of high
prevalence of thiamine deficiency in exclusively breastfed infants from
Kashmir valley strengthened the argument that thiamine deficiency in
India is far from controlled and may warrant a relook [13-18].
Furthermore, recent research has shown that even subclinical thiamine
deficiency in infancy can have a long-term negative impact on cognitive
behaviour and learning. Although in India, reported clinical cases are
only clustered around certain specific regions [7,8], it may be
reasonable to surmise a sub-clinical thiamine deficiency elsewhere in
the country. This is particularly important as the other micronutrient
deficiencies in Indian children are quite rampant [9].
Further, the overall clinical picture of thiamine
deficiency is not easy to recognize, and diagnosis is quite often missed
due to lack of awareness and non-availability of a confirmatory test,
which is expensive and technically demanding. Not surprising, the
chances of misdiagnosis is even greater in resource-poor setting
[3,36,38]. In the absence of specific diagnostic tests, a low threshold
for a therapeutic thiamine challenge is the only way to diagnose
thiamine deficiency. The practical approach is to consider thiamine
injection as a complementary resuscitation tool in infants with severe
acute conditions; more so in presence of underlying risk factors,
clinically evident malnutrition or where a dextrose-based fluid is used
for resuscitation [3,4,8].
Considering the possibility of long-term
neurodevelopmental consequences of persistent subclinical thiamine
deficiency in the infantile period, pregnant women suspected of having
thiamine deficiency should be adequately treated and the supplementation
should be continued in both mother and baby during breastfeeding.
Additionally, there is a need to sensitize health care workers in the
country to develop a high level of clinical suspicion for thiamine
deficiency and a low threshold for the administration of thiamine,
particularly when infantile thiamine deficiency is suspected. Moreover,
obstetricians need to be sensitized regarding supplementation of
thiamine in diet of at-risk pregnant and lactating mothers. Besides,
nutrition rehabilitation centers and pediatricians need to be cautioned
about the possibility of refeeding syndrome and induced- thiamine
deficiency in children with SAM.
Further, at the community level improvised strategies
like programmatic approaches to fortification, supplementation, dietary
modification (like parboiling of rice) and education, and training of
healthcare workers, are needed to improve overall thiamine status of our
population. Studies providing objective and demonstrable evidence of the
possible contribution of thiamine deficiency to infant mortality rates
in India are needed. More importantly, studies in high-risk communities
will be needed to galvanize the states to develop measures for early
diagnosis, treatment and long-term prevention of thiamine deficiency in
infancy. Lastly, additional research is needed to understand the
long-term developmental effects of subclinical thiamine deficiency and
to identify the factors that may trigger overt clinical disease in such
deficient children.
Conclusions
Infantile thiamine deficiency continues to be an
important cause of mortality and long-term morbidity in infants in
developing countries. Due to a wide range of clinical presentation
deficiency is often overlooked or mistaken for other acute problems in
the infantile period. Apart from causing infant mortality, thiamine
deficiency may have an unappreciated long-term impact on neurological
development in children with persistent subclinical deficiency during
infancy. A high index of suspicion and a low threshold for the
administration of thiamine is needed to prevent acute and long-term
complications. Additionally, there is a need to sensitize health care
workers in the country about the clinical spectrum, diagnosis and early
treatment of thiamine deficiency in infants.
Contributors: MN, RL: participated in literature
search and drafting of the manuscript; BAC: substantial contribution to
the conception of the article and supervised drafting of the manuscript.
Funding: None; Competing Interest:
None stated.
References
1. Manzetti S, Zhang J, van der Spoel D. Thiamine
function, metabolism, uptake, and transport. Biochemistry.
2014;53:821-35.
2. Bettendorff L, Wins P. Biological functions of
thiamine derivatives: focus on non-coenzyme roles. OA Biochem.
2013;1:10.
3. Hiffler L, Rakotoambinina B, Lafferty N, Martinez
Garcia D. Thiamine deficiency in Tropical Pediatrics: New Insights into
a neglected but vital metabolic challenge. Front Nutr. 2016;3:16.
4. World Health Organization. Thiamine deficiency and
its prevention and control in major emergencies. Geneva, Switzerland:
Department of Nutrition for Health and Development, World Health
Organization; 1999 (WHO/NHD/99.13)
5. Whitfield KC, Bourassa MW, Adamolekun B, Bergeron
G, Bettendorff L, Brown KH, et al. Thiamine deficiency disorders:
Diagnosis, prevalence, and a roadmap for global control programs. Ann NY
Acad Sci. 2018;1430:3-43.
6. Gibson RS. Principles of Nutritional Assessment.
2nd ed. New York, NY: Oxford University Press; 2005.
7. Rao SN, Mani S, Madap K, Kumar MV, Singh L,
Chandak GR. High prevalence of infantile encephalitic beriberi with
overlapping features of Leigh’s disease. J Trop Pediatr. 2008;54:328-32.
8. Rao SN, Chandak GR. Cardiac beriberi: often a
missed diagnosis. J Trop Pediatr. 2010; 56:284-5.
9. Swaminathan S, Edward BS, Kurpad AV. Micronutrient
deficiency and cognitive and physical performance in Indian children.
Eur J Clin Nutr. 2013;67:467-74.
10. Fattal-Valevski A, Kesler A, Sela BA,
Nitzan-Kaluski D, Rotstein M, Mesterman R, et al. Outbreak of
life-threatening thiamine deficiency in infants in Israel caused by a
defective soy-based formula. Pediatrics. 2005;115:e233-8.
11. Frank LL. Thiamine in clinical practice. J
Parenter Enteral Nutr. 2015;39:503-20.
12. Barennes H, Sengkhamyong K, René JP, Phimmasane
M. Beriberi (thiamine deficiency) and high infant mortality in northern
Laos. PLoS Negl Trop Dis. 2015;9:e0003581.
13. Bhat JI, Rather HA, Ahangar AA, Qureshi UA, Dar
P, Ahmed QI, et al. Shoshin beriberi-thiamine responsive
pulmonary hypertension in exclusively breastfed infants: A study from
northern India. Indian Heart J. 2017;69: 24-7.
14. Qureshi UA, Sami A, Altaf U, Ahmad K, Iqbal J,
Wani NA, et al. Thiamine responsive acute life-threatening
metabolic acidosis in exclusively breast-fed infants. Nutrition.
2016;32:213-6.
15. Wani NA, Qureshi UA, Ahmad K, Choh NA. Cranial
ultrasonography in infantile encephalitic Beriberi: A useful first-line
imaging tool for screening and diagnosis in suspected cases. AJNR Am J
Neuroradiol. 2016;37: 1535-40.
16. Qureshi UA, Wani NA, Ahmad K, Irshad M, Ali I.
Infantile Wernicke’s encephalopathy. Arch Dis Child. 2015;100:648.
17. Wani NA, Qureshi UA, Jehangir M, Ahmad K, Ahmad
W. Infantile encephalitic Beriberi: magnetic resonance imaging findings.
Pediatr Radiol. 2016;46:96-103.
18. Bhat JI, Ahmed QI, Ahangar AA, Charoo BA, Sheikh
MA, Syed WA. Wernicke’s encephalopathy in exclusive breastfed infants.
World J Pediatr. 2017;13:485-8.
19. Mimouni-Bloch A, Goldberg-Stern H, Strausberg R,
Brezner A, Heyman E, Inbar D, et al. Thiamine deficiency in
infancy: long-term follow-up. Pediatr Neurol. 2014;51:311-6.
20. Fattal I, Friedmann N, Fattal-Valevski A. The
crucial role of thiamine in the development of syntax and lexical
retrieval: A study of infantile thiamine deficiency. Brain.
2011;134:1720-39.
21. Harel Y, Zuk L, Guindy M, Nakar O, Lotan D,
Fattal-Valevski A. The effect of subclinical infantile thiamine
deficiency on motor function in preschool children. Matern Child Nutr.
2017;13:e12397.
22. Institute of Medicine Standing Committee on the
Scientific Evaluation of Dietary Reference Intakes and Its Panel on
Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for
Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12,
Pantothenic Acid, Biotin, and Choline. Washington, DC: Food and
Nutrition Board, National Academy Press; 1998.
23. Combs GF. The Vitamins: Fundamental Aspects in
Nutrition and Health. 5th ed. San Diego, CA: Academic Press; 2012.
24. Gropper SS, Smith JL, Groff JL. Advanced
Nutrition and Human Metabolism. 5th ed. Belmont, CA: Wadsworth; 2009.
25. Smithline HA, Donnino M, Greenblatt DJ.
Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy
subjects. BMC Clin Pharmacol. 2012;12:1-10.
26. Dudeja P, Tyagi S, Gill R, Said H. Evidence for a
carrier-mediated mechanism for thiamine transport to human jejuna
basolateral membrane vesicles. Dig Dis Sci. 2003;48:109-15.
27. Singleton CK, Martin PR. Molecular mechanisms of
thiamine utilization. Curr Mol Med. 2001;1:197-207.
28. Subramanian VS, Marchant JS, Parker I, Said HM.
Cell biology of the human thiamine transporter-1 (hTHTR1). Intracellular
trafficking and membrane targeting mechanisms. J Biol Chem.
2003;278:3976-84.
29. Thurnham, D.I. Thiamin: Physiology. Encycl Hum
Nutr. 2013:4:274-9.
30. Gangolf M, Czerniecki J, Radermecker M, Detry O,
Nisolle M, Jouan C, et al. Thiamine status in humans and content
of phosphorylated thiamine derivatives in biopsies and cultured cells.
PLoS One. 2010;5:e13616.
31. Bâ A. Metabolic and structural role of thiamine
in nervous tissues. Cell Mol Neurobiol. 2008;28:923-31.
32. Gibson GE, Zhang H. Interactions of oxidative
stress with thiamine homeostasis promote neurodegeneration. Neurochem
Int. 2002;40:493-504.
33. Ahoua L, Etienne W, Fermon F, Godain G, Brown V,
Kadjo K, et al. Outbreak of beriberi in a prison in Côte
d’Ivoire. Food Nutr Bull. 2007;28:283-90.
34. Luxemburger C, White NJ, ter Kuile F, Singh HM,
Allier-Frachon I, Ohn M, et al. Beri-beri: The major cause of
infant mortality in Karen refugees. Trans R Soc Trop Med Hyg.
2003;97:251-5.
35. McGready R, Simpson JA, Cho T, Dubowitz L,
Changbumrung S, Bohm V, et al. Postpartum thiamine deficiency in
a Karen displaced population. Am J Clin Nutr. 2001;74:808-13.
36. Abu-Kishk I, Rachmiel M, Hoffmann C, Lahat E,
Eshel G. Infantile encephalopathy due to vitamin deficiency in
industrial countries. Childs Nerv Syst. 2009;25:1477-80.
37. Rabinowitz SS. Paediatric Beri Beri. Available
from: https://emedicine.medscape.com/article/984721-overview.
Accessed October 1, 2018.
38. Lima LF, Leite HP, Taddei JA. Low blood thiamine
concentrations in children upon admission to the intensive care unit:
Risk factors and prognostic significance. Am J Clin Nutr.
2011;93:57-61.
39. Khounnorath S, Chamberlain K, Taylor AM,
Soukaloun D, Mayxay M, Lee SJ, et al. Clinically unapparent
infantile thiamine deficiency in Vientiane, Laos. PLoS Negl Trop Dis.
2011;5:e969.
40. Iqbal H, Jobayer C, Shoji Y, Rehana Y, Tahmeed A.
Features in septic children with or without severe acute malnutrition
and the risk factors of mortality. Pediatrics. 2015;135:S10.
41. Manary M, Trehan I, Weisz A. Systematic Review of
Transition Phase Feeding of Children with Severe Acute Malnutrition as
in Patients. World Health Organization. Available from:
http://www.who.int/nutrition/publica tions/guidelines/updates_management_
SAM_infantand children_review5.pdf. Accessed March 26, 2019.
42. Fuentebella J, Kerner JA. Refeeding syndrome.
Pediatr Clin North Am. 2009;56:1201-10.
43. Maiorana A, Vergine G, Coletti V, Luciani M,
Rizzo C, Emma F, et al. Acute thiamine deficiency and refeeding
syndrome: Similar findings but different pathogenesis. Nutrition.
2014;30:948-52.
44. Andersen LW, Mackenhauer J, Roberts JC, Berg KM,
Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated
lactate levels. Mayo Clin Proc. 2013;88:1127-40.
45. Kornreich L, Bron-Harlev E, Hoffmann C, Schwarz
M, Konen O, Schoenfeld T, et al. Thiamine deficiency in infants:
MR findings in the brain. AJNR Am J Neuroradiol. 2005;26:1668-74.
46. Refeeding Syndrome: Guidelines. Cape Town
Metropole Paediatric Interest Group. Available from: http://www.
mms-cpd.org/upload/guides/Nutrition%20-%20Refeeding
%20Syndrome%20Guidelines%20ECT2366.pdf. Accessed March 26, 2019.
47. Refeeding Syndrome: Prevention and Management –
SCH Practice Guideline. Australia: Sydney Children’s Hospital
Guidelines. Available from: http://www.schn.health. nsw.gov.au/_
policies/pdf/2013-7036.pdf. Accessed March 26, 2019.
48. World Health Organisation. Guideline: Updates on
the Management of Severe Acute Malnutrition in Infants and Children.
Geneva: WHO (2013).
49. Statement by Mr PS Gadhavi, Member of Parliament
and Member of Indian Delegation, on agenda item 40: follow-up to the
outcome of the special session on children at the 59th Session of the UN
General Assembly on 27 October 2004
50. Harper C. Thiamine (vitamin B1) deficiency and
associated brain damage is still common throughout the world and
prevention is simple and safe! Eur J Neurol. 2006;13: 1078-82.
|
|
|
 |
|

