|
|
|
Indian Pediatr 2013;50: 783-785 |
 |
Clinico-bacteriological Profile and Outcome of
Empyema
|
|
D Narayanappa, N Rashmi, NA Prasad, and *Anil Kumar
From the Departments of Pediatrics, and * Pediatric
Surgery, JSS Medical College and Hospital, JSS University, Mysore,
Karnataka. India.
Correspondence to: Dr D Narayanappa, No.534, ‘Sinchana’,
15th main, 5th Cross, Saraswathipuram, Mysore 570 009, Karnataka. India.
Email: [email protected]
Received: June 18, 2012;
Initial Review: November 23, 2012;
Accepted: January 28, 2013.
PII: S097475591200518
|
Empyema thoracis is a common
cause of morbidity in children. We conducted a prospective
observational study in 50 children (age 0-15 y) diagnosed with
empyema to study its clinico-bacteriological profile and outcome in
a referral hospital. Staphylococcus aureus was the most
common causative organism, most of them being MRSA, followed by
Pneumococcus and Pseudomonas. Primary video-assisted
thoracoscopy appeared to be a good mode of management with lesser
duration of hospital stay. However, the number of children
undergoing this procedure was very less, to come to any conclusion.
Key words: Empyema thoracis, MRSA,
Video-assisted thocacoscopy.
|
|
It is estimated that 0.6% of childhood pneumonias
progress to empyema, affecting 3.3 per 1,00,000 children [1].
Staphylococcus aureus is the commonest causative organism in
developing countries. There are no universally accepted guidelines for
its management in children. Treatment options include antibiotics alone
or in combination with chest tube drainage, intrapleural fibrinolytics,
VATS (video assisted thoracoscopic surgery), and open decortications
[2,3]. Not many studies are available regarding optimal management of
empyema in children. We conducted this observational study to delineate
the clinico-bacteriological profile of empyema and its outcome with
different modes of management.
Methods
This study was conducted in the Department of
Pediatrics, JSS Medical College Hospital between September 2008 to
September 2010. Fifty children admitted to J.S.S. Hospital, Mysore, with
the diagnosis of empyema in the age group between 0 to 15 years, with
diagnosis of empyema according to ICD-10 code J869 were included [4].
Children with empyema thoracis secondary to trauma/ thoracic
surgery/oesophageal rupture were excluded. Informed parental consent was
taken and relevant data were collected in a preformed proforma.
Institutional ethical clearance was obtained.
All children were subjected to investigations like
complete blood count, ESR, blood culture and sensitivity, Mantoux test,
sputum for acid fast bacilli (if available) and C-reactive protein.
Pleural fluid collected with aseptic precautions by thoracocentesis or
during the time of insertion of intercostal tube for drainage bottle was
analyzed for cell type and count, pH, glucose, LDH levels, Gram’s and
AFB stain and culture and sensitivity for aerobic bacteria. Chest X-ray,
ultrasound scan of chest and CT scan of thorax were done wherever
necessary. The mode of management in these cases (indications for) was
decided based on algorithm in Fig. 1 [2]. All the patients
were followed up after 1 month of discharge. Outcome was assessed in
terms of clinical and radiological clearance. Pulmonary function test
(PFT) was performed in children who were above 6 years of age at follow
up. Statistical methods like frequencies, descriptive, crosstabs,
chi-square test and analysis of variance were used to analyze the data,
employing the SPSS 11.0 package.
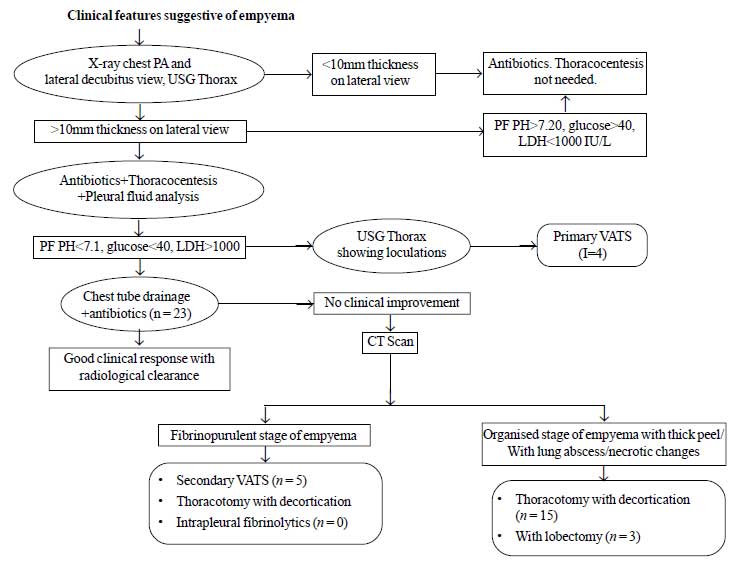 |
|
Fig. 1 Algorithm for indications for
different modes of management and details of different
management strategies.
|
Results
Out of the 50 cases studied, majority (90%) were in
the age group of 0-5+years (mean age: 3y). Males were more commonly
affected. All children had fever and cough, 35(70%) had hurried
respiration, 4 (8%) had abdominal pain, 4(8%) had chest pain and 2 (4%)
of them had ear discharge. 38(76%) of children had tachypnea, 26(52%)
had tachycardia, 46 (92%) children had dullness on percussion.
Diminished breath sounds were noted in 43(86%) children. Pleural fluid
Gram stain positivity was seen in 17 cases and isolation of organism by
pleural fluid culture was possible in only 20(40%) cases, of which 2
cases had received prior antibiotics. Contingency coefficient of pleural
fluid culture positivity between children who had received prior
antibiotics and those who had not received any, was 0.132, which was
statistically insignificant.
Of 15(30%) cases of pleural fluid culture proven
Staphylococcus aureus isolation, 4 (26.6%) were methicillin
resistant Staphylococcus aureus (MRSA). All 15 isolates were
sensitive to linezolid and amikacin, 12 (80%) to clindamycin, 5 isolates
to ceftriaxone and only 3(20%) to penicillin. 12(80%) isolates were
resistant to penicillin, followed by 5(66%) to erythromycin and 3(20%)
to ciprofloxacin. Out of 20 pleural fluid culture proven cases of
empyema, 4(8%) isolates were Pneumococci. All were sensitive to
linezolid and amikacin and 3(75%) isolates were sensitive to penicillin.
One isolate showed growth of Pseudomonas which was sensitive to
ceftazidime, cefotaxim, amikacin, ciprofloxacin and resistant to
tetracyclines.
The management of all the 50 children is shown in Fig
1. The outcome measures included were mean duration of hospital stay and
radiological clearance on follow up. The mean duration of hospital stay
in different modes of management is depicted in Table I.
TABLE I Mean Duration of Hospital Stay (Unit?Hours/days) in Different Modes of Management
|
Numbers n (%)= 50 |
Mean (SD)
|
|
CTD + antibiotics |
23 (46) |
106 (2.92) |
|
PRIM VATS |
4 (8) |
8.5 (1.73) |
|
CTD + antibiotics + Secondary VATS |
5 (10) |
15.0 (4.85) |
|
CTD + antibiotics + Thoracotomy with decortication |
15 (30) |
15.5 (5.01) |
|
CTD + antibiotics + Thoracotomy + Lobectomy |
3 (6) |
26.3 (6.11) |
|
Total |
50 (100) |
13.3 (5.7) |
|
With respect to duration of hospital stay, significant
difference was noted between primary VATS and other modes
(P<0.001). |
Of 23 cases who were treated with CTD and antibiotics
alone, 17 came for follow up after 1 month, 88.2% of them showing good
radiological clearance. 2 (11.8%) children had thickened pleura
radiologically. In children managed with Primary and Secondary VATS,
radiological clearance was 100%. Children undergoing thoracotomy with
decortications showed 93.3% radiological clearance. Children managed by
lobectomy had longer duration of hospital stay of 26.3 days
(statistically significant by Scheffe’s Post Hoc test) and all of them
developed residual scoliosis at follow-up. However there was no
statistically significant difference in radiological clearance between
different treatment strategies. Only five children in the study group
were eligible for pulmonary function test. 1 child was lost to follow
up. PFT done in 4 patients who were more than 6 years were within normal
limits.
One child aged 1 year in the study group expired due
to development of sepsis (Staphylococcus aureus was isolated in
blood culture) with DIC and meningitis.
Discussion
We observed that empyema occurs most commonly in the
under-five age group, and the clinical presentation comprise of,
tachypnea, tachycardia, dull note on percussion and diminished breath
sounds, as also noted in other studies [5-13]. Only 40% of the cases in
our study were confirmed by a positive pleural fluid culture. Girod,
et al. reported states that diagnosis made only by biochemical
criteria may represent an early empyema that may be amenable to a
nonsurgical treatment [14]. However, the yield of pleural fluid culture
also depends on the strength and quality of the culture media. Causative
organisms in our study were also similar to that reported earlier from
other developing countries [5-9]. Most of the isolates of
Staphylococcus (MRSA) were sensitive to linezolid and amikacin.
Other studies however have showed good sensitivity to third generation
cephalosporins, cloxacillin and gentamicin [6,8].
Most of the children in the study were managed by
chest tube drainage, followed by different other modes depending on
their response. Only four children underwent primary VATS, and showed
good outcome in terms of lesser duration of hospital stay and complete
radiological clearance on follow up. However, the number was too low to
come to any conclusion regarding it‘s superiority over other modes.
Studies from India and other countries showed varied outcomes with
respect to radiological clearance, chest deformity and mortality [5-10,
13], which are comparable to those from our study.
Contributors: ND: Guarantor, overall co-ordinator
and revised the manuscript for intellectual content. NR: Conception,
literature search, manuscript writing and critical revision. PNA:
Concept, data acquisition and manuscript writing. AMG: Surgical
management and literature search.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
• The most common cause of empyema in
children is Staphylococcus aureus, with increasing
prevalence of MRSA.
|
References
1. Kumar V. Epidemiological methods in ARI. Indian J
Pediatr. 1987;54:205-11.
2. Singh M, Singh SK, Choudhary SK. Management of
empyema thoracis in Children. Indian Pediatr. 2002;39:145-57.
3. Balfour-Lynn IM, Abrahamson E, Cohen G, Hartley J,
King S, Parikh D, et al. BTS guidelines for management of pleural
infection in children. Thorax. 2005;60:il-2l.
4. Light RW. Parapneumonic effusions and empyema.
In: Light RW. Pleural Diseases, 3rd edn. Baltimore: Williams
and Wilkins; 1995. p. 129-53.
5. Kumar L, Guptha AP, Mitra S, Yadev K, Pathak IC,
Walia BS, et al. Profile of childhood empyema thoracis in north
India. Indian J Med Res. 1980;72:854-9.
6. Padmini R, Srinivasan S, Puri RK, Nalini P.
Empyema in infancy and childhood. Indian Pediatr 1990;27:447-52.
7. Ghosh S, Chakraborty CK, Chatterjee BD.
Clinicobacteriological study of empyema thoracis in infants and
children. J Indian Med Assoc. 1990;88:189–90.
8. Mishra OP, Das BK, Jain AK, Lahiri TK, Sen PC,
Bhargava V. Clinico-bacteriological study of empyema thoracis in
children. J Trop Pediatr. 1993;39:380-1.
9. Baranwal AK, Singh M, Marwaha RK, Kumar L. Empyema
thoracis: a 10 year comparative review of hospitalized children from
south Asia. Arch Dis Child. 2003;88:1009-14.
10. Satpathy SK, Behera CK, Nanda P. Outcome of
parapneumonic empyema. Indian J Pediatr. 2005;72:197-9.
11. Adeyemo AO, Adeyujigbe O, Taiwo OO. Pleural
empyema in infants and children: analysis of 298 cases. J Natl Med
Assoc. 1984;76:799-805.
12. Chonmaitree T, Powell KR. Parapneumonic pleural
effusion and empyema in children: Review of a 19-year experience
1962-1980. Clin Pediatr (Phila). 1983;22:414-9.
13. McLaughlin FJ, Goldmann DA, Rosenbaum DM, Harris
GB, Schuster SR, Strieder DJ. Empyema in children: clinical course and
long term follow up. Pediatrics. 1984;73:587-93.
14. Girod CE, Neff TA. How to manage parapneumonic effusion/empyema.
J Respir Dis. 1994; 15:35-44.
|
|
|
 |
|

