Viral bronchiolitis is a common
reason for hospitalizations in infants and contributes to huge economic
burden [1, 2]. Bronchiolitis is generally seasonal and hospitalization
peaks between 3 and 6 months of life [2]. The standard treatment remains
supportive care and includes ensuring adequate oxygen exchange, fluid
intake and feeding of the infant [3, 4]. Meta analyses of data on the
most-used therapies for acute bronchiolitis namely, nebulized
bronchodilators, epinephrine, glucocorticoids and chest physiotherapy
have failed to prove any effect on relevant clinical outcomes, in
comparison with placebo except some benefits of epinephrine compared to
placebo for short-term outcomes for outpatients, particularly in the
first 24 hours of care [5-8]. Current clinical practice guidelines do
not recommend the routine use of any medication but despite the
evidence, use of ineffective therapies for bronchiolitis remains high
[9].
Recently, several investigators have reported the use
of hypertonic saline solution for infants with bronchiolitis with
substantial benefits of therapy reported by many of them [10-19]. It has
been reported to alter mucociliary clearance favorably in both normal
and diseased lungs in multiple clinical settings including bronchiolitis
[20-24]. This modality has enormous potential for cost-saving, both in
developing and developed countries, more so if it could actually reduce
length of hospitalization as suggested by a recent Cochrane review
[13]. Thus, based on the available literature, we hypothesized that 3 %
hypertonic saline would shorten length of hospital stay as compared to
0.9% saline in patients with bronchiolitis. We conducted this study to
evaluate the efficacy of nebulized 3 % hypertonic saline in children
diagnosed with clinical bronchiolitis.
Method
We designed a randomized, double-blind, controlled
trial involving infants and children aged 1 to 24 months hospitalized
with acute bronchiolitis of moderate severity. Children with clinical
presentation of viral bronchiolitis and hospitalized with a clinical
severity score 3-6 were included [24,25]. Bronchiolitis was defined by
first episode of wheezing along with prodrome of upper respiratory tract
infection including rhinorrhea, cough, and sometimes low-grade fever,
which may progress to dyspnoea. Children with obtunded consciousness,
cardiac disease, chronic respiratory disease, previous wheezing episode,
progressive respiratory distress requiring respiratory support other
than supplemental oxygen were excluded. Those having received nebulized
hypertonic saline within the previous 12 hours were also excluded. The
Institutional ethics committee of our hospital approved the study.
Signed informed consent was obtained from the parents of all children.
All patients were enrolled within 24 hours of admission to the hospital.
Computer generated random numbers were used for enrolment in consecutive
manner and patients were randomly assigned receive either 4 mL of 3%
hypertonic saline or 4 mL of 0.9% saline nebulization along with 2.5 mg
salbutamol at intervals of 4 hours, six times daily till the patient was
ready for discharge. There was no detectable difference in color, smell,
or other physical properties between 0.9% saline solution and 3%
hypertonic saline solution. The combination code of the therapeutic
package (0.9% saline vs 3% hypertonic saline) was not available
to the investigator or treating medical staff. The code was deposited
with the statistician. We used a conventional jet nebulizer with
tight-fitting face mask connected to a source of pressurized oxygen set
to a flow rate of 7 L/min through tight-fitting face mask. The
nebulization was continued till the nebulization chamber was empty.
Patients were examined by investigators at the study entry and every
day. Monitoring parameters for improvement or worsening of the condition
were measured and recorded at admission and then at 12 hourly intervals
using the clinical score described by Wang, et al. [25].
Discharge criteria included feeding well orally, no need for intravenous
fluids and supplemental oxygen, clinical severity score
3,
absence of accessory muscle use or tachypnea (respiratory rate <31
breaths/min) and oxygen saturation >92% on air. We measured length of
hospital stay from admission to time taken to reach clinical severity
score score <3.
Statistical analysis: Primary objective of
interest was to compare the length of hospital stay (time taken to reach
clinical severity score <3) and secondary objective was to compare the
improvement in clinical severity scores in hospitalized children with
acute bronchiolitis nebulized with 3% hypertonic saline and normal
saline. Reduction in length of hospital stay of 1 day was previously
proposed as being clinically significant. It was anticipated that this
would require a sample size of 113 patients in each arm. This number was
based on a pre study mean length of hospital stay of 3.5 ± 2.9 days
[17]. Each variable was scanned for normalcy of distribution.
Categorical variable were compared using the Chi-square test. All
continuous variables were compared using the paired or unpaired t-test
as appropriate. A P value <0.05 was considered statistically
significant. To examine the clinical severity scores at 12-hourly
intervals Mann-Whitney non-parametric U test was carried out in each
treatment group separately. For this analysis, P value 0.005 was
considered significant due to multiple comparisons.
Results
Study was conducted during September 2009 to December
2010 and 277 potentially eligible candidates patients with a clinical
diagnosis of bronchiolitis were admitted of which 248 successfully
completed the protocol (Fig. 1). The two study groups were
similar in baseline characteristics (Table I) including
age, sex and clinical severity score.
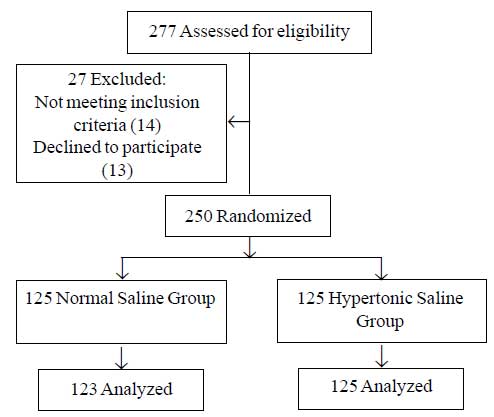 |
|
Fig.1 Participant flow diagram.
|
TABLE I Baseline Characteristics Of Study Subjects
|
Characteristics |
Hypertonic
|
Normal
|
|
saline
|
saline
|
|
N=125 |
N=123 |
|
Age, mo (mean) |
4.93 ± 4.31 |
4.18±4.24 |
|
No. of patients in different age groups |
|
|
|
1-6 mo
|
93 |
105 |
|
7-12 mo
|
24 |
13 |
|
12-24 mo
|
8 |
5 |
|
Male/female (n) |
97/28 |
92/31 |
|
Duration of symptoms* (d) |
3.6±2.87 |
3.8±2.34 |
|
Baseline O2 saturation% |
94.43±2.77 |
95.23±2.45 |
|
Mean time (h)# |
8.6±4.63 |
9.1±7.62
|
|
Clinical score^ (median) |
6 |
6 |
|
* at enrollment; #admission to enrollment; ^at
admission. |
Clinical severity scores monitored 12 hourly till
discharge (132 hours) did not show statistically significant differences
between the two groups (Fig. 2). However, it is to be
noted that the median clinical severity score at time 0 would be based
on 125 subjects whereas the data at 132 hours would be based on only 2
patients. There was no difference in mean length of hospital stay in
0.9% saline (63.93 ± 22.43 hours) & 3% hypertonic saline (63.51 ± 21.27)
groups (P=0.878). Percentage of patients remaining in each group
at 24 hourly intervals is depicted in Fig. 3. No adverse
events related to nebulized therapy were reported by the parents,
caregivers or treating medical attendants in both groups.
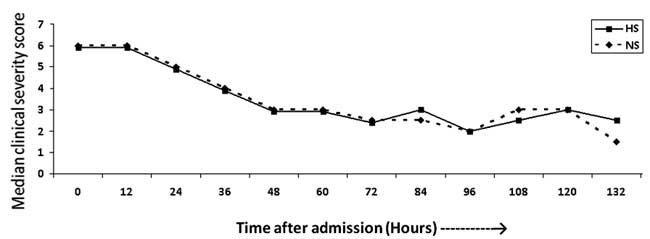 |
|
Fig. 2 Median clinical severity score
of two groups at 12-hourly intervals.
|
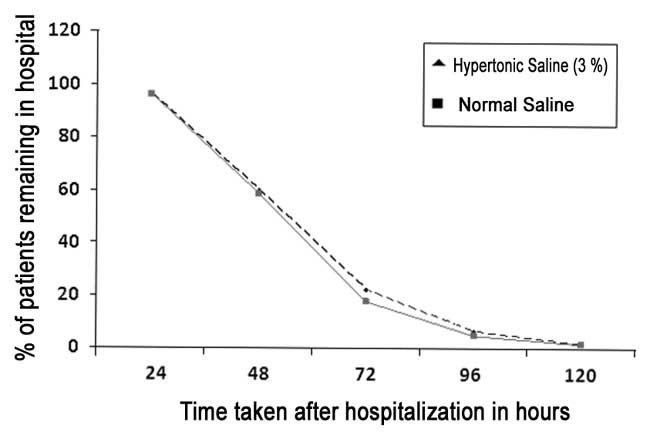 |
|
Fig.3 Patients remaining in each group
at 24-h intervals.
|
Discussion
Ours is the one of the largest studies comparing 3%
hypertonic saline and 0.9% (normal) saline nebulization in hospitalized
children with acute bronchiolitis. Both the groups were comparable in
baseline characteristics however; we did not find any advantage of
hypertonic (3%) saline over normal (0.9%) saline in terms of length of
hospital stay and clinical severity scores monitored from admission till
discharge.
Shorter length of hospital stay and lower admission
rates have been reported to be objective and clinically meaningful
measure of cost effectiveness [26, 27]. In the Cochrane meta analysis
(four studies) 24.1% shorter (mean 1.16 days, 95% CI -1.55 to - 0.77
days) length of hospital stay was reported with hypertonic saline [13].
However, one of these studies reporting the maximum reduction (-1.4
days) with hypertonic saline had longer length of stay (almost double
that of other 3 studies) in both the groups leading to heterogeneity of
data [17]. Khalid, et al. [18] have recently reported shorter
length of stay with hypertonic saline but if we look at the actual
length of hospital stay among the different groups, there is difference
of few hours only which may not be significant clinically. Revisit rate
7 days after discharge was also similar in all the groups in their study
reflecting the non superiority of hypertonic saline. Mean length of
hospital stay was similar with 3% and normal saline in our study but it
was shorter in both the groups as compared to all other investigators
[18].
Most of the studies conducted in emergency care or
outpatient settings did not show any significant advantage of hypertonic
saline over normal saline in terms of improvement in clinical severity
scores or hospitalization rates [11,12,14]. However, studies conducted
in hospitalized patients with bronchiolitis have reported better
improvement in clinical severity scores with hypertonic saline but the
magnitude of improvement differed on different treatment days varying
from 15.7 % on day 1 to 29.4 % on day 3 in hypertonic saline group
[10,13,15-17]. Concentration dependent improvement (normal saline <3%
saline <5% saline) in clinical severity scores reported by Khalid, et
al. [18] were measured immediate post nebulization but transient
improvement may not have effect on length of hospitalization [18]. We
did not find any significant difference in CS score at enrollment and
thereafter at 12 hourly intervals till discharge in both the groups.
Sood, et al. [28] have demonstrated that
increasing the volume of airway surface liquid is associated with
increased rates of mucociliary clearance in normal subjects. They found
that the change in depth of airway surface liquid after normal saline or
hypertonic saline inhalations is a function of the mass of NaCl added to
the airway surface by the aerosols of different concentrations of NaCl.
Ceiling effect of higher inhaled NaCl could be the possible reason for
inability to document any difference with hypertonic saline and normal
saline in our study.
We did not have a placebo group due to ethical
considerations as the only placebo for nebulization therapy could be NS
which itself is a treatment modality. We did not attempt virological
diagnosis as we did not have the facility. Though this is the actual
clinical scenario for management of bronchiolitis in developing
countries yet, considering the age limit we might have enrolled a few
children with diagnosis other than bronchiolitis.
To conclude, nebulized 3 % hypertonic saline is not
superior to 0.9% saline in infants and children with clinically
diagnosed acute bronchiolitis (without RSV confirmation). Further
large-scale trials are required to prove its clinical benefits before
recommending its routine use in patients with acute viral bronchiolitis.
1. Yorita KL, Holman RC, Sejvar JJ, Steiner CA,
Schonberger LB. Infectious disease hospitalizations among infants in the
United States. Pediatrics. 2008; 121:244 -52.
2. Deshpande S, Northern V. The clinical and health
economic burden of respiratory syncytial virus disease among children
under 2 years of age in a defined geographical area. Arch Dis Child.
2003; 88: 1065-69.
3. Zorc JJ, Breese HC. Bronchiolitis: Recent evidence
on diagnosis and management. Pediatrics. 2010; 125; 342-9.
4. Subcommittee on Diagnosis and Management of
Bronchiolitis. Clinical Practice Guideline: Diagnosis and Management of
Bronchiolitis, American Academy of Pediatrics. Pediatrics. 2006;
118:1774 -93.
5. Gadomski AM, Bhasale AL. Bronchodilators for
bronchiolitis. Cochrane Database Syst Rev. 2006, 3: CD001266.
6. Hartling L, Bialy LM, Vandermeer B, Tjosvold
L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis.
Cochrane Database Syst Rev. 2011;6:CD003123.
7. Patel H, Platt R, Lozano JM, Wang EE. Glucocorticoids for
acute viral bronchiolitis in infants in infants and young children.
Cochrane Database Syst Rev. 2004;3:CD004878.
8. Perrotta C, Ortiz Z, Roque M. Chest physiotherapy
for acute bronchiolitis in pediatric patients between 0 and 24 months
old. Cochrane Database Syst Rev 2006; 1: 004873.
9. Landrigan CP, Conway PH, Stucky ER, Chiang VW,
Ottolini MC. Variation in pediatric hospitalists’ use of proven and
unproven therapies: a study from the Pediatric Research in Inpatient
Settings (PRIS) Network. J Hosp Med. 2008; 3:292-8.
10. Mandelberg A, Tal G, Witzling M, Someck E, Houri
S, Balin A, et al. Nebulized 3% hypertonic saline solution
treatment in hospitalized infants with viral bronchiolitis. Chest. 2003;
123: 481-7.
11. Grewal S, Ali S, McConnell DW, Vandermeer B,
Klassen TP. A randomized trial of nebulized 3% hypertonic saline with
epinephrine in the treatment of acute bronchiolitis in the emergency
department. Arch Pediatr Adolesc Med. 2009;163:1007-12.
12. Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu
N. High volume normal saline alone is as effective as nebulized
salbutamol-normal saline, epinephrine-normal saline, and 3% saline in
mild bronchiolitis. Pediatric Pulmonol. 2010;45:41-7.
13. Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen
TP. Nebulized hypertonic saline solution for acute bronchiolitis in
infants. Cochrane Database Syst Rev. 2008;4:CD006458.
14. Sarrell EM, Tal G, Witzling M, Someck E, Houri S,
Cohen HA, et al. Nebulized 3% hypertonic saline solution
treatment in ambulatory children with viral bronchiolitis decreases
symptoms. Chest. 2002; 122: 2015-20.
15. Tal G, Cesar K, Houri S, Ballin A, Mandelberg A.
Hypertonic saline/epinephrine treatment in hospitalized infants with
viral bronchiolitis reduces hospitalizations stay: 2 years experience.
Isr Med Assoc J. 2006; 8:169-73.
16. Kuzik BA, Al Qaghi SA, Kent S, Flavin MP, Hopman
W, Hotte S, et al. Nebulized hypertonic saline in the treatment
of viral bronchiolitis in infants. J Pediatr. 2007; 151: 266 -70.
17. Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et
al. Nebulized hypertonic saline/salbutamol solution treatment in
hospitalized children with mild to moderate bronchiolitis. Pediatr Int.
2010;52:199-202.
18. Khalid AA, Sakran M, Bruce L, Davidson, Sayyed
RE, Mahjoub H, et al. Nebulized 5% or 3% hypertonic or 0.9%
saline for treating acute bronchiolitis in infants. J Pediatr.
2010;157:630-4.
19. Luo Z, Fu Z, Liu E, Xu X, Fu X, Peng D, et al. A
randomized controlled trial of nebulized hypertonic saline treatment in
hospitalized children with moderate to severe viral bronchiolitis. Clin
Microbiol Infect. Clin Microbiol Infect. 2011;17:1829-33.
20. Daviskas E, Anderson SD, Gonda I, Eberl S, Meikle
S, Seale JP, et al. Inhalation of hypertonic saline aerosol
enhances mucociliary clearance in asthmatic and healthy subjects.
European Resp J. 1996;9:725-32.
21. Wark P, McDonald V, Jones A. Nebulised hypertonic
saline for cystic fibrosis. Cochrane Database Syst Rev. 2007;4:CD001506.
22. Tarran R, Donaldson S, Boucher RS. Rationale for
hypertonic saline therapy for cystic fibrosis lung disease. Semin Respir Crit
Care Med. 2007;28: 295-302.
23. Kellett 2005 Kellett F, Redfern J, Niven RM.
Evaluation of nebulised hypertonic saline (7%) as an adjunct to
physiotherapy in patients with stable bronchiectasis. Resp Med.
2005;991:27-31.
24. Shoseyov D, Bibi H, Shai P, Ahoseyov N, Shazberg
G, Hurvitz H. Treatment with hypertonic saline versus normal saline
nasal wash of pediatric chronic sinusitis. J.Allergy and Clin Immunol.
1998;101:602-5.
25. Wang EE, Milner RA, Navas L, Maj H. Observer
agreement for respiratory signs and oximetry in infants hospitalized
with lower respiratory infections. Am Rev Resp Dis. 1992;145:106-9.
26. Ralston S, Hill V, Martinez M. Nebulized
hypertonic saline without adjunctive bronchodilators for children with
bronchiolitis. Pediatrics. 2010;126:e520-5.
27. Horner D, Bartram T, Jenner R Morton R. Can the
efficacy of hypertonic saline in bronchiolitis truly be assessed with a
short-term primary outcome? Arch Pediatr Adolesc Med. 2010; 164:395.
28. Sood N, Bennett WD, Zeman K, Brown J, Foy C,
Boucher RC, et al. Increasing concentration of inhaled saline
with or without amiloride: effect on: mucociliary clearance in normal
subjects. Am J Respir Crit Care Med. 2003;167:158-63

