|
|
|
Indian Pediatr 2010;47: 694-701 |
 |
Home-based Rehabilitation of Severely
Malnourished Children in Resource Poor Setting |
|
Deepak Patel, Piyush Gupta, Dheeraj Shah and Kamlesh Sethi*
From the Departments of Pediatrics and *Dietitics,University
College of Medical Sciences (University of Delhi) and
Guru Teg Bahadur Hospital, Dilshad Garden, Delhi 110 095, India.
Correspondence to: Dr Dheeraj Shah, Associate Professor,
Department of Pediatrics, University College of Medical Sciences and
associated GTB Hospital, Dilshad Garden, Delhi 110 095, India.
Email: [email protected]
|
|
Abstract
Objective: To evaluate the feasibility and
outcome of home-based rehabilitation of severely malnourished children.
Design: Prospective and observational.
Setting: Rehabilitation at home (16 weeks)
following initial assessment or/and stabilization at hospital.
Participants: Thirty-four severely malnourished
(weight for length <70% of WHO reference) children between the ages of 6
months to 5 years.
Intervention: Initial assessment of the patient
was done in hospital. Those with complications or loss of appetite were
admitted in hospital and managed as per WHO guidelines. After discharge,
they were managed at home using home based diets. Those without
complications and with preserved appetite were directly eligible for
home-based rehabilitation. Follow up was done in hospital up to 16
weeks. Dietary intake, anthropometry and morbidities were recorded
during follow-up.
Results: Of the enrolled 34 children, 19 children
were admitted in hospital and 15 children were sent home after initial
assessment in hospital. Five did not clear the initial stabilization
phase (2 died, 3 left hospital). Finally 29 children qualified for home
based rehabilitation out of which 26 completed 16 week follow-up. During
the home based management phase, the reported mean (±SD) calorie intake
increased from 100 (±5) kcal/kg/d at entry point to 243 (±13) kcal/kg/d
at 16 weeks (P=0.000). Similarly, reported protein intake
increased from 1.1 (±0.3) g/kg/d to 4.8 (±0.3) g/kg/d (P=0.000).
During hospital stay (n=19), children had weight gain of 9.0
(±5.3) g/kg/d, while during home based follow up (n=29), weight
gain was 3.2 (±1.5) g/kg/d only. During home based rehabilitation, only
3 (11.5%) children had weight gain of more than 5 g/kg/d by the end of
16 weeks. Weight for height percent increased from an average (±SD) of
62.9% (±6.0%) to 80.3% (±5.7%) after the completion of 16 weeks (P=0.000).
Thirteen (45%) children recovered completely from malnutrition achieving
a weight for length of >80% whereas 15 (51.7%) recovered partly
achieving weight for length >70%. There was no death during the home
stabilization.
Conclusion: Home based management using home
prepared food and hospital based follow up is associated with
sub-optimal and slower recovery.
Key words: Community health services, Nutritional support,
Protein energy malnutrition.
|
|
To rationalize the management of severely
malnourished children, World Health Organization (WHO) proposed guidelines
which state that a child with complications should be treated in hospital
until the weight for length improves above 90%(1,2). However, this is
seldom feasible because of bed shortage in hospitals and budgetary
constraints. Prolonged hospital stay also carries the risk of nosocomial
infections leading to increased mortality. Admission of all children with
severe malnutrition is thus not operationally feasible, and hence
home-based management is an unavoidable alternative for a significant
proportion of these subjects. Preliminary evidence from Bangladesh and
Africa suggests that this alternative may be acceptable, cost-effective,
and reduce morbidity and mortality(3-8). In a recent statement, WHO
suggested that uncomplicated forms of severe acute malnutrition can be
treated in the community with ready-to-use therapeutic food (RUTF) or
therapeutic diets using locally available nutrient dense foods under
careful monitoring until they have gained adequate weight(9). However, the
guidelines regarding early discharge from the hospital followed by home
based management are lacking. The studies evaluating outcome of those
discharged early from the hospital or those managed at home are scarce.
The present study was thus conducted to evaluate the outcome and
feasibility of home based rehabilitation of severely malnourished
children. We hypothesized that it is possible to attain recovery and
reduce mortality with home based rehabilitation.
Methods
The study was conducted between November 2006 to
February 2008 in a prospective and observational manner. The setting was a
tertiary care hospital in Delhi, North India. The hospital predominantly
caters to poor urban population living in resettlement colonies and slums,
and also rural families from neighbouring state of Uttar Pradesh. The
study protocol was approved by Institutional Research Board, including
ethical clearance. The study procedure was fully explained to the
parents/caregivers, and informed written consent was obtained from the
primary caregiver.
Inclusion criteria
All consecutive children (6 months to 5 years) with
severe malnutrition presenting to the Pediatric out-patient department (OPD)
or emergency of hospital were eligible for inclusion. Severe malnutrition
was defined as weight for height (or length) <70% of median(2). WHO
multicentric growth standards were used as reference criteria(10).
Children were eligible for home management if: (i)
mother or caretaker was not full-time employed, (ii) family was
residing within 5 km of hospital premises, (iii) mother or
caretaker was trainable to provide home-based diet, and (iv)
family was financially able to provide the recommended home-based diet.
Children having other diseases incriminated as cause of severe
malnutrition, including cerebral palsy, congenital heart disorders,
hemolytic anemia, malignancies, known metabolic disorders, known
malabsorption syndromes, chromosomal malformations, or chronic renal and
hepatic disorders, were excluded.
Baseline data collected included demographic details
and presence of associated symptoms, including fever, vomiting or
diarrhea. Occupation, education and monthly income of parents were
recorded and a socio-economic status was assigned based on revised
Kuppuswamy classification(11). General hygiene of the household was
assessed in terms of source of drinking water, water storage practices,
and practice of hand washing. Clinical examination included anthropometry
(weight, length, mid-arm circumference, chest circumference and head
circumference), general physical examination and systemic examination. The
weight was recorded in the nude on an electronic weighing scale (Goldtech,
India) to the nearest 5 g. Length was recorded using an infantometer, and
head, chest and mid-upper arm circumference were recorded using non-strechable
measuring tape using standard techniques(12). The same observer recorded
all the measurements. Venous blood was drawn for estimation of hemoglobin,
blood sugar, albumin and serum electrolytes. Cultures of the blood, and
urine were taken. Chest X-ray and other relevant investigations
were done as and when required.
Assessment and Management
Initial assessment of the patient was done in hospital.
Those with complications or loss of appetite were admitted in hospital and
managed as per WHO guidelines as adapted by Indian Academy of Pediatrics (IAP)(3).
After discharge, they were managed at home using home based diets. Those
without complications and with preserved appetite were directly eligible
for home-based rehabilitation after initial assessment in hospital.
Initiation of cautious feeding: Feeding was started
with starter F-75 made at hospital kitchen using whole milk, sugar, oil
and water as per recommended formula(1). As no mineral mix or
micronutrient mix was available, potassium, magnesium and zinc were given
separately as described above. Initially enough starter was given to
provide 100 kcal/kg/day and 1-1.5 g protein/kg/day with 130 mL/kg/day of
fluid (when the child had severe edema 100 mL/kg/day was given).
Breastfeeding was continued as usual wherever possible. Initially, the
child was given feed more frequently with low volume, then gradually
frequency of feeds was decreased and volume per feed was increased. As the
patient started to regain appetite, the starter feed was changed with
home-made food. Mother was counseled to provide type, frequency and
quantity of food. Monitoring for amount of feed offered and left over,
frequency of vomiting and watery stool were done.
Discharge: Child was considered for
discharge when (i) the appetite had returned (easily consuming more
than 80% of recommended feeds orally), (ii) child had started
gaining weight (gain of at least 5 g/kg/day for 3 consecutive days), (iii)
immunization had been initiated, (iv) all acute complications had
been treated, (v) micronutrient supplementation was initiated, and
(vi) mother had been counseled for home-based care(13).
Home-Based Rehabilitation
All children directly enrolled for home-based
rehabilitation and those discharged from hospital were eligible for
home-based rehabilitation.
Nutritional support: Home based foods were advised
to provide 150 kcal/kg/day and 2-3 g/kg/day of proteins. A diet chart was
provided using home-based energy dense foods like besan-panjiri,
khichdi, parantha enriching them with jaggery and oil.
Energy dense feeding was gradually increased so as to provide
approximately 150-220 kcal/kg/day and proteins 4-5 g/kg/day. Mother was
given advice about type of food, quantity of food, and feeding frequency.
No external support for procuring or making food was provided to the
families. Daily multivitamin supplement was continued till 16 weeks.
Sensory stimulation and emotional support: Mother
was counseled to give tender loving care to the child and to provide
cheerful stimulating environment. Child was provided with toys as a part
of structured play therapy. Toys like ring on a string, rattle and drum,
in and out toy with blocks, posting bottle and doll were given.
Follow-up
Frequency of follow-up visits were: (i) 2
contacts/week separated by at least 48 hours in first two weeks, (ii)
once a week for 3-8 weeks, (iii) and from 8 weeks till 16 weeks,
every 4 weeks. At each visit, dietary intake was recorded by
recall-method, and detailed general physical and systemic examination was
done. Mother was recounselled about type, quantity and frequency of food
to be given. Any medical problem identified during visits was treated.
Measurement of weight, length, head circumference, chest-circumference and
mid-arm circumference was done at each visit. Investigations of all
children were repeated at 4 weeks and at 16 weeks. The child was stated to
have recovered completely if child achieved weight for length >80% at the
end of 16 weeks. The child was stated to be partially recovered if
the weight for length remained between 70% to 80% after 16 weeks of
follow-up. A weight gain of >5g/kg/d was defined as an acceptable weight
gain(1,2).
Statistical analysis: Descriptive analysis was
carried out for most outcomes. Calorie intake, protein intake, weight and
weight-for-height percent at 16 weeks were compared from baseline by
paired t test. Data were analyzed using SPSS 12.0 software.
Results
34 severely malnourished children formed the subjects
for present study (Fig 1). 41% children were less
than 1 year of age, and 62% were females. Of 34 children, only 3 (8.8%)
were completely immunized, only 5 (14%) children were exclusively
breastfed for 6 months, and around two-third were bottle-fed. Nearly half
(53%) had access to safe water supply and two-thirds (64%) had facility of
separate toilet. Anthropometry of study subjects at presentation (n=34)
is given in Table I. At first assessment, all
children were anemic (Hb <11 g/dL). Thrombocytopenia (platelet count <150
× 10 9 /L) was observed in 6 (17.6%),
hypoglycemia in 3 (8.9%), hyponatremia (Na+ <130 mEq/L) in 4 (11.8%),
hypernatremia (Na+ >150 mEq/L) in 7 (21%), hypokalemia (K+ <3.5 mEq/L) in
11 (32%), and hyperkalemia in (K+ >5.5 mEq/L) 4 (11.8%) children. Blood
culture at presentation was positive in 7 (21%) children, urine culture
was positive in 2 (5.9%), and stool demonstrated ova/cyst in 3 (8.8%)
children. At 16 weeks, hematological, biochemical and microbiological
profile was normal in all children.
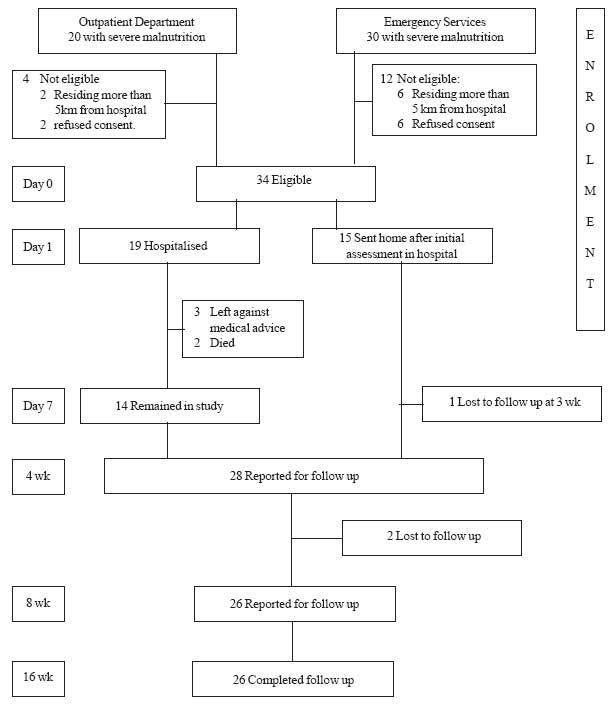 |
|
Fig. 1 Study flow chart.
|
TABLE I
Baseline Characteristics and Anthropometry of Study Subjects at Presentation
|
Characteristics |
Admitted (n=19) |
Directly Recruited (n=15) |
Total (n=34) |
| |
mean (SD) |
mean (SD) |
mean (SD) |
| Age (mo) |
18.7 (10.0) |
21.9 (14.4) |
20.1 (12.1) |
| Males (%) |
8 (42.1) |
5 (33.3) |
13 (38.2) |
| Weight (kg) |
5.2 (1.3) |
5.1 (1.6) |
5.1 (1.4) |
| Weight for age (%) |
47.5 (7.2) |
48.1 (7.5) |
47.9 (7.4) |
| Length (cm) |
70.3 (7.4) |
69 (9.9) |
69.7 (8.4) |
| Length for age (%) |
85.9 (6.2) |
85.1 (5.9) |
85.5 (6.1) |
| Weight for length (%) |
62.5 (6.4) |
65 (3.8) |
63.7 (5.5) |
| Chest circumference (cm) |
39.7 (3.7) |
40.1 (4.1) |
39.8 (3.8) |
| Mid-arm circumference (cm) |
9.3 (1.2) |
9.3 (0.9) |
9.3 (1) |
Of 34 patients, 19 were admitted and treated in
hospital. The mean (±SD) duration of hospitalization was 5.4 (±2.2) days
(median: 6 d; IQR: 4-7 d). Two children had edema during presentation,
which settled on day 5 and 8 of admission. Two children died during
hospital stay and another 3 children left against medical advice. Thus
fourteen children thus were finally discharged. Fifteen children were not
hospitalized and sent home after initial assessment in hospital. These 29
children were eligible for home based rehabilitation. Of these, 26
children completed the follow up (Fig.1).
Dietary intake : The mean calorie and protein
intake of study subjects who completed 16 weeks follow-up (N=26) at
enrolment, day 3, day 7, 3 week, 6 week, 12 week and 16 week is shown in
Table II. During the home based management phase, the mean
(±SD) calorie intake increased from 100 (±5) kcal/kg/d at enrolment to 243
(±13) kcal/kg/d at 16 weeks (P<0.001). Similarly, protein intake
increased from 1.1 (±0.3) g/kg/d to 4.8 (±0.3) g/kg/d (P<0.001).
TABLE II
Changes in Calories and Protein Intake, Weight, and Weight for Length During Follow Up (N=26)
| Days of
enrolment |
Calories (kcal/kg/day) |
Protein (g/kg/day) |
Weight (kg) |
Weight
for height/length |
| Baseline
|
100.0 (5.0) |
1.1 (0.3) |
4.97 (1.4) |
62.9
(6.0) |
| 3 days |
117.3 (4.5) |
1.3 (0.2) |
5.07 (1.4) |
64.2
(5.8) |
| 7 days |
140.0 (13.2) |
1.6 (0.2) |
5.20 (1.4) |
65.6
(5.6) |
| 3 week |
161.5 (17.8) |
2.4 (0.3) |
5.45 (1.4) |
68.7
(5.6) |
| 6 weeks |
184.6 (18.1) |
3.3 (0.2) |
5.79 (1.4) |
72.7
(5.7) |
| 12 weeks |
225.2 (20.6) |
4.4 (0.4) |
6.36 (1.4) |
77.1
(4.8) |
| 16 weeks |
242.8* (13.1) |
4.8* (0.3) |
6.70* (1.5) |
80.3*
(5.7) |
*Significantly (P<0.001) higher than baseline values by paired t test. Values represent mean (SD).
|
Anthropometry: The mean (±SD) weight (kg) and
weight for length (%) of study subjects at enrolment, day 3, day 7, week
3, week 6, week 12 and week 16 is also shown in Table II.
Weight gain during hospital stay was 9.0 (±5.3) g/kg/d, while during
home-based rehabilitation, average weight gain was 3.2 (±1.5) g/kg/d.
During home based rehabilitation, only 3 (11.5%) children achieved weight
gain of more than 5 g/kg/d, while 26 (89.5%) children had weight gain of
less than 5 g/kg/d.
The recovery was complete in 13 (44.8%) children. Of
these, 2 children achieved
³80%
weight for length at 6 weeks, 1 child at 7 weeks, 1 child at 8 weeks, 3 at
12 weeks and 6 children at 16 weeks follow-up. The mean (±SD) weight for
length in these children at 16 weeks was 85% (±3.9%). The mean (±SD) time
needed to achieve 80% weight for length was 12 (±3) weeks. Of those who
recovered completely, 4 children went up to achieve >90% of weight for
length by the end of 16 week follow-up. Partial recovery occurred
in 15/29 (51.7%) children who achieved weight for length of between
70-80%. One child (3.5%) continued to have severe malnutrition even after
16 weeks. Fig. 2 compares the number of children with
% weight for length (<70%, 70-80%, >80%) at 4, 8, 12, and 16 weeks of
follow up.
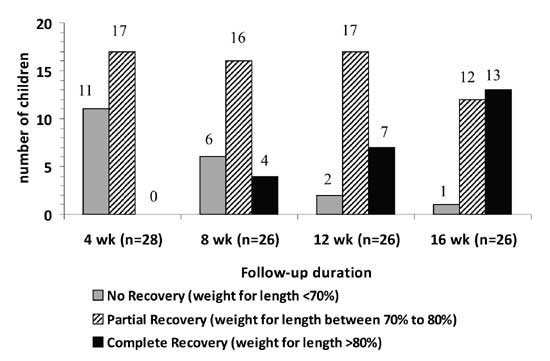 |
|
Fig.2 Number of children achieving complete
or partial recovery during follow-up. |
Three children were lost to follow-up (1 at 3 weeks,
and 2 at 4 weeks). However, all these 3 children had crossed the 70%
weight for length barrier before opting out of the study, and thus were
classified as having partial recovery.
Discussion
The present study assessed the efficacy of home-based
rehabilitation after initial assessment or following discharge from a
hospital. We aimed to assess the outcome of home-based management using
home-based foods in real life situation as no external monetary support or
food was provided during this phase. No home visits were done. Using this
strategy, majority of children failed to achieve weight gain of more than
5 g/kg/d; the standard criteria for effectiveness as defined by WHO(1).
Also, less than half of children achieved >80% weight for length after
follow-up of 16 weeks. The mean weight gain during home based
rehabilitation was also less than weight gain during hospital stay.
The mean weight gain in our study (3.2 g/kg/d) was less
than that by Gaboulaud, et al. (3) (9.7 g/kg/d), Ashraf, et al.
(4) (6 g/kg/d), and Khanum, et al.(7) (4 g/kg/d). Also,
the mean duration for achieving weight for length >80% was more in our
study in comparison to earlier studies from Bangladesh(4,7). The reasons
for early recovery and more weight gain in former studies could be the
involvement of salaried health care workers from community in giving
proper training and advice. These workers had regular home visits, whereas
in our study, home visits during follow-up were not done. Non-availability
of ready to use therapeutic food (RUTF) and no external support to the
families in form of food or money could be a possible reason of inadequate
weight gain in our study. In our patients, weight gain was inadequate
during follow-up in most, despite history of consuming adequate calories
and proteins from home-based foods. This could be attributed to the poor
reliability of dietary recall method as well as lack of a mechanism of
monitoring their food preparation and techniques of feeding in our study.
It is possible that the foods were not sufficiently energy dense, and the
adequate numbers of feedings were not provided due to social constraints.
Linear programming analysis of diets from Africa and Bangladesh has
suggested that several home prepared diets for severely malnourished
children do not achieve the nutrient density required for successful
rehabilitation(14). Persistent infections were unlikely as we could not
find any such evidence during follow up, and no child on home-based
management died.
In a recent review by Ashworth(15), thirty-three
studies of community-based rehabilitation were examined and summarized for
the period of 1980-2005; eleven (33%) programs were considered effective.
The two indicators of effectiveness that were set for this review were
mortality <5% and weight gain
³5
g/kg/day. Of the sub-sample of programs reported since 1995, 8 of 13 (62%)
were effective. Most effective programs utilized RUTF or provided
mechanisms for procuring/preparing energy dense foods. None of the
programs operating within routine health systems without external
assistance was effective.
A potential limitation of our study was that we did not
evaluate the outcomes in a comparative manner, and thus the efficacy of
the management was not directly compared with hospital care or any other
effective approach. The sample size was also small to do any subgroup
analysis. The strength of our study was that it was done in realistic
scenario without actually providing the food or financial support. This
has important operational implications as most facilities managing
severely malnourished children in India currently do not provide
therapeutic nutrition, and only rely on nutritional advice/counseling.
We conclude that home-based management (directly or
following early discharge from hospital) using home prepared food and
hospital based follow up is associated with sub-optimal and slow recovery.
There should be effective monitoring system and mechanism for providing
energy dense foods/ RUTF to strengthen the community based management of
severely malnourished children. Effectiveness of community-based
rehabilitation may require careful planning and additional resources,
including nutrition educators. Provision of RUTF might help in
strengthening the implementation process but its cost, logistics of
procurement and distribution, sustainability, and consequences of
withdrawal would need to be carefully considered. Future research should
include comparative evaluation of different strategies in a controlled
manner and operational research to strengthen the existing home and
hospital based approaches.
Contributors: PG and DS conceptualized and
supervised the study. DP and KS collected the data and followed up the
patients. DP and KS prepared the initial draft of manuscript which was
revised by PG and DS. All authors approved the final manuscript.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
•
Home management of
uncomplicated severe malnutrition is a feasible option. Ready-to-use
therapeutic food (RUTF) is an effective option in controlled
settings.
What this Study Adds?
•
Home based
management using home prepared food and hospital based follow up is
associated with sub-optimal and slower recovery.
|
References
1. Ashworth A, Khanum S, Jackson A, Schofield C.
Guidelines for the Inpatient Treatment of Severely Malnourished Children.
Geneva: World Health Organization; 2003.
2. Bhatnagar S, Lodha R, Choudhary P, Sachdev HPS, Shah
N, Narayan S, et al. IAP Guidelines 2006 on Hospital based
Management of Severely Malnourished Children (adapted from WHO
guidelines). Indian Pediatr 2007; 44: 443- 461.
3. Gabouland V, Dan-Bouzoua N, Brasher G, Fedida G,
Gergonne B, BrownV. Could nutritional rehabilitation at home complement or
replace centre-based therapeutic feeding programmes for severe
malnutrition? J Trop Pediatr 2007; 53: 49-51.
4. Ashraf H, Ahmed T, Hossain MI, Alam NH, Mahmud R,
Kamal SM, et al. Day-care management of children with severe
malnutrition in an urban health clinic in Dhaka, Bangladesh. J Trop
Pediatr 2007; 53: 171-178.
5. Orach C, Kolsteren P. Outpatient care of severely
malnourished children. Lancet 2002; 360: 1800-1801.
6. Ashworth A, Khanum S. Cost effective treatment of
severely malnourished children: what is best approach? Health Policy Plan
1997; 12: 115-121.
7. Khanum S, Ashworth A, Hulty SR. Controlled trial of
three approaches to the treatment of severe malnutrition. Lancet 1994;
344: 1728-1732.
8. Bredow M, Jackson A. Community-based, effective, low
cost approach to the treatment of severe malnutrition in rural Jamaica.
Arch Dis Child 1994; 71: 297-303.
9. Community-Based Management Of Severe Acute
Malnutrition: A Joint Statement by the World Health Organization, the
World Food Programme, the United Nations System Standing Committee on
Nutrition and the United Nations Children’s Fund. World Health
Organization; 2007.
10. de Onis M, Garza C, Onyango AW, Martorell R. WHO
Child Growth Standards. Acta Paediatr 2006; 95 (Suppl 450): 1-104.
11. Mishra D, Singh HP. Kuppuswamy’s socio-economic
status scale: A revision. Indian J Pediatr 2003; 70: 273-274.
12. Physical Status: The Use and Interpretation of
Anthropometry- Report of a WHO Expert Committee. WHO Technical Report
Series; 854. Geneva: World Health Organization; 1995.
13. Gupta P, Shah D, Sachdev HPS, Kapil U. National
Workshop on Development of Guidelines for Effective Home Based Care and
Treatment of Children Suffering from Severe Acute Mal-nutrition. Indian
Pediatr 2006; 43: 131-139.
14. Ferguson EL, Briend A, Darmon N. Can optimal
combinations of local foods achieve the nutrient density of the F100
catch-up diet for severe malnutrition? J Pediatr Gastroenterol Nutr 2008;
46: 447-452.
15. Ashworth A. Efficacy and effectiveness of
community-based treatment of severe malnutrition. Food Nutr Bull 2006;
27(3 Suppl): S24-S48.
|
|
|
 |
|

