|
|
|
Indian Pediatr 2020;57: 335-342 |
 |
Novel Coronavirus 2019 (2019-nCoV) Infection:
Part II - Respiratory Support in the Pediatric Intensive Care
Unit in Resource-limited Settings
|
|
Manu Sundaram 1, Namita Ravikumar 2,
Arun Bansal 2, Karthi Nallasamy 2,
Basavaraja GV 3, Rakesh Lodha 4, Dhiren
Gupta 5, Marti Pons Odena 6, Ashwath Ram
RN 7, Muralidharan Jayashree 2 for the
Intensive Care Chapter of Indian Academy of Pediatrics
From 1Division of Critical Care Medicine,
Sidra Medicine, Doha, Qatar; 2Division of
Pediatric Critical Care, Department of Paediatrics, Advanced
Paediatrics Centre, Postgraduate Institute of Medical
Education and Research (PGIMER), Chandigarh, India 3Pediatric
Intensive Care Unit, Indira Gandhi Institute of Child
Health, Bangalore, Karnataka, India; 4Department
of Pediatrics, All India Institute of Medical Sciences,
Delhi, India; 5Pediatric Intensive Care Unit, Sir
Ganga Ram Hospital, Delhi, India; 6Department of
Pediatric Intensive Care, Sant Joan de Due Hospital,
Barcelona, Spain; and 7Department of Pediatric
Intensive Care, Manipal Hospital, Bangalore, India.
Correspondence to:Dr Arun Bansal, Professor, Department of
Pediatrics, Advanced Pediatrics Centre, Postgraduate
Institute of Medical Education and Research, Chandigarh,
India.
[email protected]
Received: March 26, 2020;
Initial review: March 28, 2020;
Accepted: March 29,
2020.
Published online: March 29, 2020;
PII: S097475591600152
|
|
The 2019-novel coronavirus predominantly
affects the respiratory system with manifestations ranging
from upper respiratory symptoms to full blown acute
respiratory distress syndrome (ARDS). It is important to
recognize the risk factors, categorize severity and provide
early treatment. Use of high flow devices and non-invasive
ventilation has been discouraged due to high chances of
aerosol generation. Early intubation and mechanical
ventilation areessential to prevent complications and
worsening, especially in resource-limited settings with very
few centers having expertise to manage critical cases.
Hydrophobic viral filter in the ventilator circuit minimizes
chances of transmission of virus. Strategies to manage ARDS
in COVID-19 include low tidal volume ventilation with
liberal sedation-analgesia. At the same time, prevention of
transmission of the virus to healthcare workers is extremely
important in the intensive care setting dealing with severe
cases and requiring procedures generating aerosol. We,
herein, provide guidance on non-invasive respiratory
support, intubation and management of ARDS in a child with
COVID-19.
Keywords: 2019-
nCoV, Aerosol generation, ARDS, Management, Pandemic, SARI.
|
|
Novel coronavirus 2019 (2019-nCoV) infection has been declared a
pandemic by the World Health Organization (WHO). We elaborated the
epidemiology, preparedness of intensive care units, clinical course,
intensive care needs and complication of patients with Coronavirus
disease (COVID-19) in a previous article [1]. In this write-up, we will
focus on the respiratory manifestations, progression and intensive care
management of respiratory compli-cations of COVID-19. As we learn more
about the 2019-nCoV (novel coronavirus) and the impact this has had on
the patients and health care workers (HCW) globally, the focus has
shifted to safety of the HCW so that the patients can be treated
appropriately and kept safe. This is based on the lessons learned from
previous epidemics and mitigating steps to reduce risks to HCW. Most of
the following suggestions are based on expert opinionon providing safe
care in these challenging times.
RESPIRATORY DISEASE DUE
to 2019 nCoV INFECTION
Clinical Course
The most
common presentation is short history of prodrome with myalgias, malaise,
cough and low-grade fever. As per the case series from China, only
40-70% of the pediatric patients have fever as an initial presentation
[2-4]. During the second week of illness, progression of the disease
gradually leads to difficulty in breathing. Reports from China have
suggested that it takes an average of 8 days for the development of
dyspnea and 9 days for the onset of pneumonia/pneumonitis [5].
Investigations
CDC does not currently recommend
chest radiography (CXR) or computed tomography (CT) to diagnose COVID-19
[6]. Viral testing remains the only specific method of diagnosis and has
been discussed in detail in part-I [1]. Confirmation with the viral test
is required, even if radiologic findings are suggestive of COVID-19 on
CXR or CT scan [7].
Differential Diagnosis
The clinical presentation and findings on chest imaging in COVID-19
are not specific.The clinical presentation of COVID-19 overlaps with
other infections like influenza, respiratory syncytial virus (RSV),
adenovirus, human meta-pneumovirus, non COVID-19 coronavirus, atypical
organisms (mycoplasma, chlamydia) and bacterial infections. It is not
possible to differentiate COVID-19 from these infections clinically or
through routine laboratory tests. In the context of pandemic and local
transmission setting in, the travel history will become irrelevant.
There are some radiological and hematological findings that may help
indicate COVID-19, even though they are not very specific [1].
Classification of Severity
Severity of illness
is based on the presenting symptoms and has been discussed previously
[1]. Patients can shed RNA from 1-4 weeks after symptom resolution, but
it is unknown if the presence of RNA equals presence of infectious
virus. As per current guidelines, COVID-19 patients are “cleared” of
isolation once they have 2 consecutive negative RNA tests collected >24
hours apart. This practice may not be clinically possible in our setting
due to various constraints. Therefore, keeping them in isolation for
longer duration is the key.
MANAGEMENT OF HYPOXEMIC
RESPIRATORY FAILURE
One of the key considerations during
management is mitigating risk to health care workers. Hypoxemia can be
present due to impaired respiratory functions in COVID-19. Oxygen
supplementation treatment can correct hypoxemia and relieve secondary
organ damage caused by hypoxemia[8]. The management of children with
Severe acute respiratory illness (SARI) in COVID is similar to any other
viral pneumonia with ARDS but with strict precautions to reduce risk of
transmission[9].
Protection From Aerosol
All aerosol generating procedures/events require donning of personal
protective equipment which includes N95 mask, goggles or face shield,
cap, full sleeve gown and shoe cover (Table I) [10].
Where possible, a nebulizer may be replaced with an MDI and spacer for
administration of bronchodilators. NIV generates droplets >10 µm in size
and most fall on local surfaces within 1-meter distance. Learning from
droplet dispersion studies, HCWs who are providing NIV, chest
physiotherapy or working within 1 meter of an infected patient should
have a high level of respiratory protection [11-13].
Table I Aerosol Generating Events and Procedures in the Intensive Care Unit
|
Aerosol generating events |
Procedures vulnerable to aerosol generation | |
Inadequate seal during |
Laryngoscopy | |
NIV or HFNC |
Intubation | |
Nebulization |
Front of neck access | |
Endotracheal suction |
Laryngoscopy | |
CPR prior to intubation |
Bronchoscopy | |
Extubation | | |
Coughing/sneezing | | |
NIV-non-invasive ventilation; HFNC – High flow nasal cannula. |
Oxygen Therapy
Oxygen therapy is necessary for patients with oxygen saturation
(SpO2) less than 90% and/or with signs of respiratory distress.
It has been noted that many elderly patients with severe
hypoxemia may not have obvious symptoms of respiratory distress
[14]. It is pertinent that the evaluation of all children with
respiratory symptoms should include pulse oximetry. Low flow
oxygen devices are recommended as high flow devices have the
potential for risk of spread through aerosol generation. Nasal
cannula at flows of 2-4 L/min is a good choice for milder forms
of SARI. A triple layer mask should be used to cover the mouth
and nose of the patient over the nasal cannula, especially
during transport, unless the child does not tolerate [15].
Heated Humidified High Flow Nasal Cannula
(HHHFNC/HFNC)
HFNC therapy can be useful in
special situations for hypoxia. A flow of 2-3 mL/kg with FiO2
targeted to SpO2 is used. However, it is necessary that when
patient is on HFNC interface, HCW are wearing optimal airborne
PPE and child is managed in negative pressure rooms,if available
[16]. In infants, while HFNC is being given they can be placed
in an oxygen hood to minimize dispersion. Surviving Sepsis
Guidelines recommend HFNC in milder cases of adult SARI [17].
However, no such guidelines are there for children. HFNC should
be tried for a maximum of 1-2 hours. Signs of improvement are
decrease in heart rate and respiratory rate by 10-20%, decrease
in FiO2 requirement to less than 50% and improvement in oxygen
saturations.
Patients with worsening hypercapnia,
acidemia, respiratory fatigue, hemodynamic instability or those
with altered mental status should be considered for early
invasive mechanical ventilation.
Non-invasive
Ventilation
Over the last two decades, the use
of non-invasive ventilation (NIV) is increasing in children with
viral illness and the rates of intubation are reducing. At the
same time there is paucity of literature regarding the use of
NIV in respiratory pandemics.
In a Chinese observational
study in adults of the SARS outbreak, it was shown that NIV was
effective in preventing the use of endotracheal intubation in
70% of patients because of early initiation of NIV. In this
study, none of the HCW acquired SARS from the patients. This was
attributed to NIV being applied in a negative-pressure
environment with strict PPE regime and close monitoring of the
HCW involved [18]. In another study from Toronto during SARS,
the use of NIV was discouraged after clinicians contracted the
disease when a patient was intubated following NIV failure [19].
There-fore, some clinicians consider NIV is contra-indicated for
acute respiratory failure due to airborne respiratory diseases
unless it is used in a negative-pressure isolation room and
strict precautions are taken [19].
After the two viral
pandemics, most of the professional societies including the
European Respiratory Society, European Society of Intensive Care
Medicine, and American Association for Respiratory Care have
recommended against NIV use to treat acute respiratory failure
due to H1N1 influenza, particularly in severely ill patients.
Thus, NIV is accepted as a high-risk procedure that should be
used cautiously because of possible spread of infection [20-23].
Routine use of NIV is not recommended in COVID-19. It
should be used only in selected patients with hypoxemic
respiratory failure. Ideally, negative pressure single rooms are
preferable for patients on NIV. However, in an outbreak of such
a magnitude, some professional societies recommend keeping a
distance of at least two meters between two beds. Due to the
high percentage of failure with NIV and the rapid progression of
the hypoxemic failure due to COVID-19, all patients receiving
NIV need a clear plan for treatment failure.
Selection of
interface is the key for success and protection of the HCWs.
Preferred interfaces are helmet, total face mask and oro-nasal
non-vented masks.Risks of NIV include delayed intubation, large
tidal volumes and injurious trans-pulmonary pressures. Limited
data suggest a high failure rate in patients with other similar
viral infections such as MERS-CoV [24].
PaO2/FiO2is a
sensitive and accurate indicator of oxygenation function on NIV
and can be used to define the severity of ARDS once the patient
has been on a PEEP of 5 cm for a minimum of 30 minutes. Invasive
ventilation must be considered if PaO2/FiO2 ratio is below 300.
In the absence of an ability to do an arterial blood gas, the
SpO2/FiO2 can also be used to identify oxygenation failure as
long as the FiO2 has been titrated to get saturations between
92%- 97%.
The most recent World Health Organization (WHO)
interim guidance on management of the novel-CoV has also
recommended the use of NIV for mild cases of ARDS without
hemodynamic instability [8].
Conventional ventilators
with NIV option having double lumen tubing is a safer option
than NIV ventilator with single lumen tubing requiring
exhalation port to washout the CO2. Antiviral/Antibacterial
filters should be attached to the exhalation limb of the circuit
to reduce environmental contamination. Alternatively, when these
options are not available, home ventilators with built-in oxygen
blender or transport ventilators can provide adequate mechanical
ventilation.
Bubble CPAP
In
situations where both non-invasive and invasive mechanical
ventilation are not available, bubble nasal CPAP (commercial or
indigenous) may be used for newborns and children with severe
hypoxemia as these are readily available alternative in
resource-limited settings.To minimize environmental
contamination the infant could be placed in an oxygen hood to
reduce droplets.
These patients should be on continuous
monitoring and cared for by experienced personnel capable of
performing endotracheal intubation in case the patient acutely
deteriorates or does not improve after a short trial (about 2
hours).
Patients with known contraindications for NIV
like moderate/severe ARDS with PaO2/FiO2 ratio below 200,
hemodynamic instability, multi-organ failure, or abnormal mental
status should receive invasive ventilation from the very
beginning.
Intubation
During the
previous SARS epidemics in China and Singapore, infection rates
were higher in doctors and nurses carrying out endotracheal
intubation [relative risk (95% CI)-13.3 (2.99–54.04)] [20]. In
an observational study of influenza-A and influenza-B in exhaled
breath, viral RNA was seen in one-third of infected patients and
99% of particles had a diameter of <5 µm when sampled during
tidal breathing [25]. Studies have demonstrated that particles
<10 µm in diameter are more likely to cause infection in the
lower respiratory tract [9,10]. Corona-virus virions (or
‘particles’) are spherical particles with diameters of
approximately 125 nm (0.125 µm) [26].
Tracheal
intubation should be performed as early as possible for patients
with aPaO2/FiO2 ratio <300, worsening trend of the SpO2/FiO2
ratio <200, worsening respiratory distress, high concentration
(>60%) of oxygen on HFNC or multiple organ dysfunction.
Table II Intubation Trolley and Tray and Modifications for Use in COVID-19 Patients
|
Equipment (size appropriate) |
Specific for COVID-19 patients | |
Laryngoscope with blade |
Video laryngoscope is preferred to increase the distance between the health worker and patient | |
Endotracheal tube |
Micro-cuffed and cuffed tubes to minimize aerosol as well as leak in acute respiratory distress syndrome (ARDS) | |
Suction catheter |
Closed suction to minimize contact with secretion, aerosol release & de-recruitment | |
Hydrophobic viral filter |
Used between the ambu bag and mask as well as in the ventilator circuitat the expiratory end | |
Oxygen & ventilation delivery |
For pre-oxygenation, use non-rebreathing mask or a flow inflating device (Jackson Rees) Ensure devices adequate mask seal Avoid bagging if using self-inflating bag | |
Drugs – Sedo-analgesia & neuromuscular blockade |
Use liberal sedation &neuromuscular blockade to avoid coughing and ultra-rapid sequence intubation | |
Adjuncts |
Stylet, Bougie and second-generation laryngeal mask airway (LMA) devices readily available if initial plan fails0 |
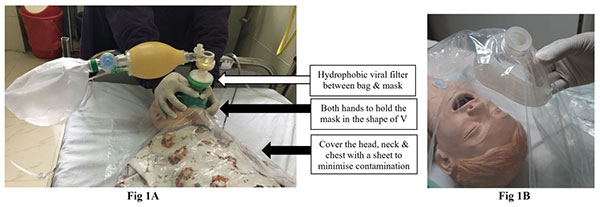 |
| Fig. 1 (a) The
assembly of bag, viral filter and mask along with
plastic sheet to minimize aerosol; (b) Preparing the
sheet with an opening for the mask. |
Preparation : Prepare the plan, ready the
equipment and set-up the ventilator prior to intubation
(Table II). At least three personnel are needednamely,
airway operator, airway assist and a nurse for medication. The
most experienced person should intubate to ensure minimum number
of attempts to decrease aerosol generation. Wherever possible,
usedisposable equipment. Video laryngoscopy is ideal to protect
the intubating HCW from operating too close to the airway
(Fig.2). If equipment or expertise isnot
available, take measures to reduce droplets during the procedure
using a plastic sheet (Fig. 1).
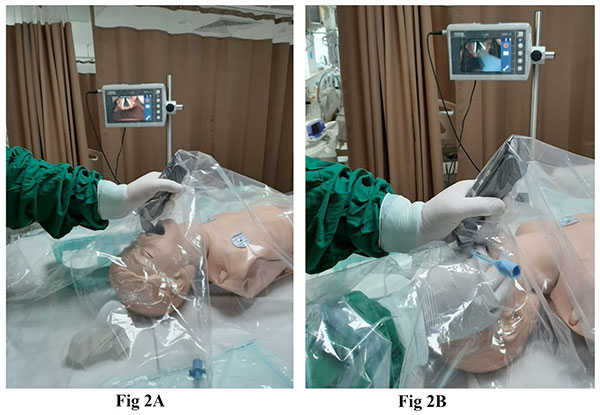 |
| Fig. 2 (a) Video-laryngoscope
assisted intubation; (b) shows a sheet covering the face
and chest during intubation. |
Pre-medication: Use benzodiazepine (midazolam
0.1-0.2 mg/kg) with opioid (fentanyl 2-3 µg/kg) combination for
sedation and analgesia. Short acting neuromuscular blockers like
rocuronium is preferred (if unavailable, use a higher dose of
vecuronium or atracurium as per availability).
Pre-oxygenation: After a quick assessment for anatomically
difficult airway, pre-oxygenation is carried out with
non-rebreathing mask (NRM) or tight-fitting face mask attached
to a self-inflating ambu-bag with 100% oxygen for 5 minutes. A
hydrophobic viral filter between the mask and ambu-bag is
recommended and some units cover the head, neck and chest with
transparent plastic apron/sheet to prevent aerosol contamination
(Fig.1). Avoid bag and mask ventilation (BMV)
to limit aerosol and if needed, use low tidal volume with lesser
breaths.
Intubation: Cuffed endotracheal tubes (ETT) must
be used in all ages and cuff needs to be inflated immediately
following intubation. Disposable ventilator circuit with a viral
filter attached at the expiratory limb (between circuit and
machine) is used. Heat moisture exchanger (HME) is preferred for
humidification. Ventilator should be in ‘stand-by’ mode and only
to be turned on after connected to the patient. Prior to
connecting to ventilator, the ETT can be clamped or attached to
a viral filter. Closed suction (inline suction catheters) is
preferred to prevent aerosol generation. If not available, open
suction may be performed with aerosol precautions and after
adminis-tering a dose of short acting neuromuscular blocking
agent.
Invasive Mechanical Ventilation
Lung protective mechanical ventilation (MV) is recommended
strategy for management of acute hypoxemic respiratory failure.
SSC guidelines in adults recommend low tidal volume strategy
(4-8mL/kg), limiting plateau pressures to <30 cmH2O and using
higher PEEP (>10 mm Hg) [17]. Permissive hypercapnia is well
tolerated and may reduce volu-trauma. Viral filters should be
utilized, and circuits should be maintained for as long as
allowable (as opposed to routine changes) (Table III).
Table III Strategies in the Management of Acute Respiratory Distress Syndrome in COVID-19
|
Management similar to any ARDS |
Specific with respect to COVID | |
Lung protective ventilation |
Early invasive ventilation – avoid HFNC and NIV | |
Tidal volume 4-6 mL/kg |
Avoid steroids – may prolong viral shedding | |
Limit Plateau pressure <28 cm H2O |
Use liberal neuromuscular blockadeto prevent coughing | |
PEEP start with 7-10 and titrate to 15 cm H2O |
Proning involves risk of exposure to HCW and best avoided | |
Limit FiO2<60% with permissive hypoxemia |
Avoid nebulization | |
Avoid fluid overload (FO) - target FO <5% | | |
Sedo-analgesia titrated to sedation scores | | |
Early enteral nutrition – initiate within 24 hours and achieve full feeds by 48 hours | | | |
Transfusion trigger hemoglobin<7 g/dL if stable hemodynamics and oxygenation | | |
Target hemoglobin 10g/dL in refractory hypoxemia or unstable shock | | |
NIV – non-invasive ventilation; HFNC – high flow nasal cannula; HCW-healthcare worker; PEEP – peak end-expiratory pressure. |
Prone Ventilation
Prone ventilation is a recommended strategy in adults with
PaO2/FiO2 <150 to improve lung mechanics and oxygenation.Patient
is usually kept prone for12-16 hours. Prone ventilation can be
ceased once PaO2/FiO2is > 150 for more than 4 hours in the
supine position. However, in children and resource-limited
setting, due to limited availability of HCWs and PPEs, it may
not be possible to prone the child and may unnecessarily
increase the risk of infection to the healthcare workers.
Fluid Management
To reduce
pulmonary exudation and improve oxyge-nation, the fluid balance
should be strictly controlled while ensuring adequate end-organ
perfusion. Fluid restriction to 70-80% maintenance is necessary
to prevent fluid overload.
Strategies to Prevent
Ventilator-Associated Pneumonia (VAP)
VAP
bundled strategies should be strictly implemented as per
recommendations [27].
Weaning and Extubation
Once the patient’s PaO2/FiO2 is more than 300 the
neuromuscular blockade and sedatives must be weaned and
discontinued. Extubation should be performed if the patient is
considered ready for extubating to nasal O2 as post-extubation
NIV is avoided where possible.Aerosol precautions are essential
during extubation. Few units practice extubating using a plastic
bag over the face with a tight seal after inflating with oxygen
(Fig. 4) or some units use a transparent large
plastic sheet over the face and chest to capture droplets from
coughing and suctioning. Post-extubation, the need for HFNC or
NIV can be assessed while reducing monitoring.
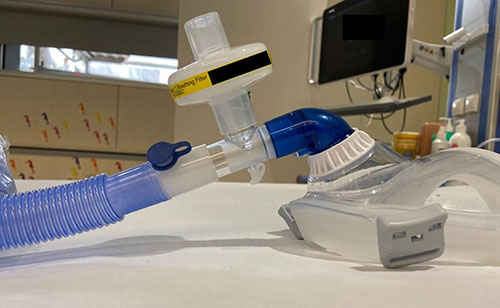 |
| Fig. 3 Use of
expiratory filter in single limb NIV tubing (use a
non-vented mask). |
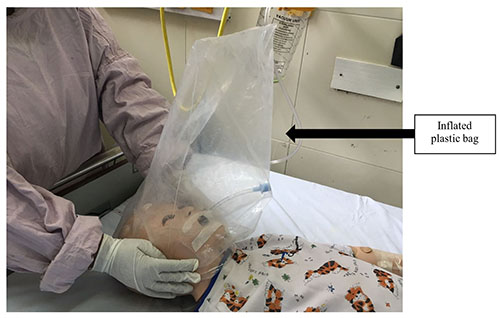 |
| Fig. 4 Covering the
face with a plastic bag or a sheet, to prevent aerosol
spread during extubation. |
Various professional bodies have
given their recommendations for respiratory support in
pediatrics and adult [8,15,17,28] and these are summarized in
Table IV.
Table IV Summary of Respiratory Support Guidelines for COVID-19 Patients
|
WHO |
SCCM |
PICS |
ANZICS | |
[7] |
[16] |
UK [27] |
[14] | |
HFNO with precautions |
+/– |
+ |
+ |
+ | |
NIV with precautions |
+/– |
+/– |
+ |
– | |
Invasive ventilation |
+ |
+ |
+ |
+ | |
WHO – World health organization, SCCM – Surviving sepsis campaign, PICS UK – Pediatric intensive care society UK, ANZICS – Australian and New Zealand Intensive Care Society. |
CONCLUSION
SARI is the most common presentation of COVID-19 and requires
intensive care support. Low flow oxygen devices are preferred to
high flow devices to prevent aerosol generation. Early
intubation and mechanical ventilation are recommended to delay
progression and need for emergent intubation, which poses
significantly higher risk of transmission of infection to HCW.
Use of HFNC and NIV is to be avoided routinely and if necessary,
a full PPE with aerosol precautions is a must. Management of
ARDS includes lung protective ventilation with liberal
sedation-analgesia and avoidance of steroids.
Contributors: MS, NR, AB, KN: substantial contribution to the
conception and design of the work, anddrafting the work; GVB,
RL, DG, MPO, ARRN, MJ: substantial contributions to the
acquisition and interpretation of data for the work,and revising
it critically for important intellectual content.All authors
gave final approval of the version to be published, and agree to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Funding: None; Competing interest: None stated.
REFERENCES
1. Ravikumar N, Nallasamy K, Bansal
A, Angurana SA, Basavaraja GV, Sundaram M, et al for the
Intensive Care Chapter of Indian Academy of Pediatrics. Novel
coronavirus 2019 (2019-nCoV) infection: Part I - Preparedness
and management in the pediatric intensive care unit in
resource-limited settings. Indian Pediatr. 2020; 57:324-34.
2. Xia W,Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT
features in pediatric patients with COVID 19 infection:
Different points from adults. Available
fromhttps://onlinelibrary.wiley.com/doi/full/10.1002/ppul.24718.
Accessed on March 29, 2020.
3. Chen C, Cao M, Pend L, Guo
X, Yang F, Wu W, et al. Coronavirus Disease-19 Among Children
outside Wuhan, China (Internet). Lancet Child AdolescMed. 2020.http://dx.doi.org/10.2139/ssrn.3546071.
4. Henry BM, Oliveira MHS. Preliminary epidemiological
analysis on children and adolescents with novel coronavirus
disease 2019 outside Hubei Province, China: an observational
study utilizing crowdsourced data
(pre-print).medRxiv. 2020.03.01.20029884. Available from:
https://www.medrxiv.org/content/10.1101/2020.03.01.
20029884v2.Accessed March 29, 2020.
5. Huang C, Wang Y,
Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
2020;395:497-506.
6. American College of Radiology. ACR
Recommendations for the use of Chest Radiography and Computed
Tomography (CT) for Suspected COVID-19 Infection. Updated March
19, 2020. Available from:
https://www.acr.org/Advocacy-and-Economics/ACR-Position
Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection.
Accessed on March 29, 2020.
7. Center of Disease Control.
Interim Guidelines for Collecting, Handling, and Testing
Clinical Specimens from Persons Under Investigation (PUIs) for
Coronavirus Disease 2019 (COVID-19) [internet]. Available from
https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html.
Accessed March 29, 2020.
8. World Health
Organization. Clinical management of severe acute respiratory
infection (SARI) when COVID-19 disease is suspected: Interim
guidance, 13 March 2020. World Health
Organization. https://apps.who.int/iris/handle/10665/331446. Accessed
March 29, 2020.
9. Murthy S, Gomersall CD, Fowler RA.
Care for critically ill patients with COVID-19. JAMA. 2020
[early online]. Available from:
https://jamanetwork.com/journals/jama/fullarticle/2762996.
Accessed March 29, 2020.
10. Brewster DJ, Chrimes NC, Do
TBT, Fraser K, Groombridge CJ, Higgs A, et al. Consensus
statement: Safe airway society principles of airway management
and tracheal intubation specific to the COVID-19 adult patient
group. Med J Aust. 2020 [pre-print].
https://www.mja.com.au/journal/2020/212/10/consensus-statement-safe-airwaysociety-principles-airway-management-and.
Accessed on March 29, 2020.
11. Simonds A, Hanak
A, Chatwin M, Morrell M, Hall A. Evaluation of droplet
dispersion during non-invasive ventilation, oxygen therapy,
nebuliser treatment and chest physiotherapy in clinical
practice: Implications for management of pandemic influenza and
other airborne infections. Health Technol Assess 2010;14:131-72.
12. Brigdes CB, Kuehnert MJ, Hall CB. Transmission of
influenza: Implications for control in health care settings.
Clin Infect Dis. 2003;37:1094-101.
13. Beggs CB. The
airborne transmission of infection in hospital buildings: Fact
or fiction? Indoor Built Environ. 2003;12:9-18.
14. Xie
J, Tong Z, Guan X, Du B, Qui H, Slutsky AS. Critical care crisis
and some recommendations during the COVID-19 epidemic in China.
Intensive Care Med. 2020. Available from:
https://link.springer.com/article/10.1007/s00134-020-05979-7.
Accessed March 25, 2020.
15. Australian and New Zealand
Intensive Care Society. ANZICS COVID-19 Guidelines. Melbourne;
2020. Available from:
https://www.anzics.com.au/wp-content/uploads/2020/03/ANZICS-COVID-19-Guidelines-Version-1.pdf.
Accessed March 25, 2020.
16. Zhu N, Zhang D, Wang W, Li
X, Yang B, Song J, et al., for the China Novel Coronavirus
Investigating and Research Team. A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med.
2020;382:727-33.
17. AlhazzaniW, Mřller MH, Arabi YM,
Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign:
Guidelines on the management of critically Ill adults with
coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020
[unedited accepted proofs].
https://www.esicm.org/wp-content/uploads/2020/03/SSC-COVID19-GUIDELINES.pdf.
Accessed March 29, 2020.
18. Cheung TMT, Yam LYC, Lau
ACW, Kong BMH, Yung RWH. Effectiveness of noninvasive positive
pressure ventilation in the treatment of acute respiratory
failure in severe acute respiratory syndrome. Chest.
2004;126:845-50.
19. Poutanen SM, Low DE, Henry B,
Finkelstein S, Rose D, Green K, et al. Identification of severe
acute respiratory syndrome in Canada. N Engl J Med.
2003;348:1195-2005.
20. Fowler RA, Guest CB, Lapinsky
SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe
acute respiratory syndrome during intubation and mechanical
ventilation. Am J RespirCrit Care Med. 2004;169:1198-202 .
21. World Health Organization. Infection and control of
epidemic-and pandemic-prone acute respiratory diseases in health
care. WHO interim guidelines. World Health Organization;2007.
Available from: http://www.who.int/csr/resources/publications/
WHO_CD_EPR_2007_6/en/. Accessed March 19, 2020.
22. Nava
S, Schreiber A, Domenighetti G. Noninvasive venti-lation for
patients with acute lung injury or acute respi-ratory distress
syndrome. Respir Care. 2011;56:1583-8.
23. Nin N, Soto
L, Hurdato J, LorenteJA,Buroni M, Arancibia F, et al. Clinical
characteristics and outcomes of patients with 2009 influenza A
(H1N1) virus infection with respiratory failure requiring
mechanical ventilation. J Crit Care. 2011;26:186-92.
24.
Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A,
et al. Clinical course and outcomes of critically ill patients
with Middle East respiratory syndrome coronavirus infection. Ann
Intern Med. 2014;160:389-97.
25. Fabian P, McDevitt JJ,
DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza virus
in human exhaled breath: an observational study. PLoS One.
2008;3:e2691
26. Zhu N, Zhang D, Wang W, Li X, Yang B,
Song J, et al. A novel coronavirus from patients with pneumonia
in China, 2019. N Engl J Med. 2020;382:727-33.
27.
Hellyer TP, Ewan V, Wilson P, Simpson AJ. The Intensive Care
Society recommended bundle of interventions for the prevention
of ventilator-associated pneumonia. J Intensive Care Soc.
2016;17:238-43.
28. Pediatric Intensive Care Society.
Updated PICS guidance on management of critically ill children
with Covid-19 infection. Available from:
https://picsociety.uk/wp-content/uploads/2020/03/PICS-Covid-19-guidance-v4.0-14Mar2020-1.pdf.
Accessed March 25, 2020.
|
 |
|

