|
|
|
Indian Pediatr 2020;57: 324-334 |
 |
Novel Coronavirus 2019 (2019-nCoV) Infection:
Part I - Preparedness and Management in the Pediatric Intensive
Care Unit in Resource-limited Settings
|
|
Namita Ravikumar1, Karthi Nallasamy1,
Arun Bansal1, Suresh Kumar Angurana1, Basavaraja
GV2, Manu Sundaram3, Rakesh Lodha4,
Dhiren Gupta5, and Muralidharan Jayashree1 for the
Intensive Care Chapter of Indian Academy of Pediatrics
From Division of
Pediatric Critical Care, Departments of Paediatrics, 1Advanced
Paediatrics Centre, Postgraduate Institute of Medical Education
and Research (PGIMER), Chandigarh, India; 2Paediatric
Intensive Care Unit, Indira Gandhi Institute of Child Health,
Bangalore Karnataka, India; 3Division of Critical
Care Medicine, Sidra Medicine, Doha, Qatar; 4Department
of Pediatrics, All India Institute of Medical Sciences, New
Delhi, India; and 5Pediatric Intensive Care Unit, Sir
Ganga Ram Hospital, New Delhi, India.
Correspondence
to:Dr Arun Bansal, Professor, Department of Pediatrics, Advanced
Pediatrics Centre, Postgraduate Institute of Medical Education
and Research, Chandigarh, India.
[email protected]
Received: March
26, 2020;
Initial review: March 28, 2020;
Accepted:
March 31, 2020.
Published online: March 29, 2020;
PII: S097475591600151
|
First reported in China, the 2019
novel coronavirus has been spreading across the globe. Till
26 March, 2020, 416,686 cases have been diagnosed and 18,589
have died the world over. The coronavirus disease mainly
starts with a respiratory illness and about 5-16% require
intensive care management for acute respiratory distress
syndrome (ARDS) and multi-organ dysfunction. Children
account for about 1-2% of the total cases, and 6% of these
fall under severe or critical category requiring pediatric
intensive care unit (PICU) care. Diagnosis involves a
combination of clinical and epidemiological features with
laboratory confirmation. Preparedness strategies for
managing this pandemic are the need of the hour, and involve
setting up cohort ICUs with isolation rooms. Re-allocation
of resources in managing this crisis involves careful
planning, halting elective surgeries and training of
healthcare workers. Strict adherence to infection control
like personal protective equipment and disinfection is the
key to contain the disease transmission. Although many
therapies have been tried in various regions, there is a
lack of strong evidence to recommend anti-virals or
immunomodulatory drugs.
Keywords: COVID-19, Guideline,
Pandemic, SARI, Treatment.
|
|
E
The year 2020 started with the emergence of the 2019 novel
corona virus (2019-nCoV) as a threat to the world; shortly afterwards
the World Health Organization (WHO) declared it a pandemic. Having begun
in China, globalization and travel led its spread all over the globe,
overwhelming the healthcare resources and resulting in high mortality
and morbidity. About 5% of adults, especially those with co-morbidities,
were critically ill and required intensive care unit (ICU) care [1].
People of all ages were found to be susceptible but severe illness was
rare in children [2]. Most of the experience of critical care management
of pediatric patients with coronavirus disease 2019 (COVID-19) is
derived from the affected children of present epidemic in China, as well
as from the previous coronaviral outbreaks viz. Severe acute respiratory
syndrome (SARS) and Middle East respiratory syndrome (MERS). We write
this review as a guidance statement for preparedness and managing
children with suspected or confirmed COVID-19 requiring intensive care
in a resource-limited setting like India.
BURDEN
Global: Till March 26, 2020, a total of 416,686 confirmed cases from
197 countries with 18,589 deaths have been reported by WHO. China has
reported the maximum cases with a total of 81,869, followed by Italy
with 69,176 cases. However, mortality is more in Italy with 6,820 (9.9%)
deaths followed by China having 3,287 (4%) deaths. The United States of
America has surpassed Spain and Germany over the last few days with
51,914 cases and 673 deaths [3].
Indian scenario: A total of 606 cases with 10
deaths have been reported from India as on March 26, 2020 as
reported by the WHO. Among these cases, only one child from
Kerala has been tested positive.
EPIDEMIOLOGY
The 2019-nCoV belongs to a group of enveloped positive-sense
RNA viruses in the family, Coronaviridae with 4 genera viz.,
alpha, beta, gamma and delta. Human coronaviruses (HCoV) belong
to alpha and beta genus and are mostly implicated in endemic
respiratory infection with mild severity [4]. However, the novel
coronaviruses infecting humans namely, SARS-CoV, MERS-CoV and
SARS-CoV-2 are believed to have originated from bats with few
intermediate hosts like civet cats, camels and pangolins [5].
RNA viruses mutate faster than DNA viruses, single-stranded
viruses mutate faster than double-strand virus, and genome size
appears to correlate negatively with mutation rate.
Transmission Characteristics
It is
speculated that it originated in bat (genetic character matches
to bat corona virus) then it got transmitted to pangolins, or
scaly anteaters. Humans seem to be accidental host who got this
virus from pangolins in Wuhan seafood market. Human to human
transmission of COVID-19 started in Wuhan city, Hubei Province
of China where it was initially labelled as ‘Pneumonia of
unknown etiology’. Epidemiological investigation of early
transmission dynamics revealed that 55% of the cases of COVID-19
during December, 2019 were linked to the hunan seafood wholesale
market. The mean incubation period has been reported to be 5.2
days with the 95th centile being 12.5 days. The main modes of
transmission include droplet and fomites followed by airborne
transmission. Reproduction number of nCoV-19 is between 2.2 to
3.6, which is comparable to SARS-CoV but higher than
MERS-CoV[6].
Less severe affection in children: Children
less than 10 years of age accounted for 1% of the total cases
[1]. The median age among pediatric cases was 6.7 years [7]. The
lesser proportion of severe cases among children has been
attributed to lesser opportunities for exposure and immaturity
of angiotensin converting enzyme 2 receptors, which are proposed
to be the binding sites for coronaviruses [8,9].
Case Fatality Rate
The overall case fatality
rate as per China Centre for Disease Control and Prevention
(CDC) is 2.3%, which is much lower compared to SARS (9.6%) and
MERS (34%) but significantly higher compared to the latest H1N1
influenza pandemic (0.001 – 0.007%)[1]. However, as per WHO, the
global case fatality rate is as high as 4.4% with absolute
number of deaths already higher than the total fatality of SARS
and MERS combined [10]. The case fatality reported from Italy is
7.2% which has gone up to 9.8% as per WHO (as on March 26, 2020)
[11].
CLINICAL MANIFESTATIONS
The common clinical features reported in the critically ill
patients include fever (98%), cough (77%), dyspnea (63%),
malaise (35%), myalgia, headache, nausea, vomiting and diarrhea
[12]. A prospective study from China involving 171 children with
confirmed COVID-19 reported fever (41%) with a median duration
of 3 days (1-16), cough (48%), pharyngeal erythema (46%)
tachypnea (28%) and diarrhea (8.8%). The cohort had 15%
asymptomatic, 19% upper respiratory infection, and 65%
pneumonia. Only 3 children (1.7%) required care and mechanical
ventilation. All three of them had comorbidities, and one died
[7].
ICU Requirements in COVID
The severe and critical categories require admission and
management in ICU. Among adults, 7% of patients admitted with
SARS-CoV-2 pneumonia required ICU care. The mean age of these
ICU patients was 60 years with male: female ratio of 2:1 and 50%
had chronic illness. Majority had Multi-organ dysfunction
syndrome (MODS) with ARDS (67%), acute kidney injury (29%),
liver dysfunction (29%) and cardiac injury (23%). Of the ICU
admissions, 71% required mechanical ventilation, 35% vasoactive
support, 17% renal replacement therapy and 11% ECMO. Mortality
was as high as 61% among the critically ill [12]. As per
unpublished data from Italy, 16% of admitted patients with
COVID-19 needed ICU care [13]. In the Chinese pediatric cases,
5.9% of all pediatric cases belonged to the severe or critical
categories. Based on the experience in managing
community-acquired pneumonia, high-risk pediatric population
includes children with underlying conditions such as congenital
heart disease, broncho-pulmonary hypoplasia, airway/lung
anomalies, severe malnutrition, and immunocompromised state;
however, more information is needed in the setting of COVID-19
[2].
DIAGNOSIS
Case definitions
for suspected, probable and confirmed COVID-19 cases as given by
WHO are in Box I [16]. The largest series on
children analyzing suspected and confirmed COVID cases is from
the electronic data base of Chinese CDC [17]. Cases were
suspected based on the presence of clinical features and
exposure history. They also identified high-risk cases and
categorized into groups based on severity (Box II).
|
Box I World Health Organization
Case Definitions for Coronavirus Disease 19 (COVID-19) |
Suspect case
A. A patient with acute respiratory illness
(fever and at least one sign/symptom of respiratory
disease (e.g., cough, shortness of breath), AND with no
other etiology that fully explains the clinical
presentation AND a history of travel to or residence in
a country/area or territory reporting local transmission
(See situation report) of COVID-19 disease during the 14
days prior to symptom onset.
OR
B. A
patient with any acute respiratory illness AND having
been in contact with a confirmed or probable COVID-19
case (see definition of contact) in the last 14 days
prior to onset of symptoms
OR
C. A patient
with severe acute respiratory infection (fever and at
least one sign/symptom of respiratory disease (e.g.,
cough, shortness breath) AND requiring hospitalization
AND with no other etiology that fully explains the
clinical presentation.
Probable case
A suspect case for whom testing for COVID-19 is
inconclusive. Inconclusive being the result of the test
reported by the laboratory
Confirmed case
A person with laboratory confirmation of
COVID-19 infection, irrespective of clinical signs and
symptoms
Source: World Health Organization
[16]. |
|
Box II Risk Stratification and Severity
Categorization for Coronavirus Disease-19 (COVID-19) |
High risk cases
Clinical features
Fever, respiratory/ digestive symptoms, fatigue
Laboratory tests
Leukopenia, lymphopenia, high C-reactive protein
Radiology
Abnormal chest ray
Severity categorization
Asymptomatic infection clinical or
radiological features but tested positive
Mild
Upper respiratory or gastrointestinal symptoms and signs
Moderate
Clinical/radiological features of lower respiratory
involvement
Severe
Presence of dyspnea or hypoxemia requiring oxygen,
refusal
to feed, altered sensorium
Critical
Organ dysfunction including Acute respiratory distress
syndrome (ARDS), shock, encephalopathy, myocardial
dysfunction, coagulation dysfunction and acute kidney
injury
Modified From Dong, et al. [17]. |
| |
Laboratory testing of suspected cases is
based on clinical and epidemiological factors. Screening
protocol should be adapted to local situation and may change
with the evolution of the outbreak scenario in the local
population. Recent testing strategy in India (as on March 20,
2020) given by ICMR is as per algorithm in Fig. 1[18].
Specimen handling for molecular testing would require Biosafety
2 (BSL-2) or equivalent facilities. Attempts to culture the
virus require minimum of BSL-3 facilities [19].
 |
| Fig. 1 Testing
strategy for suspected cases as per Indian Council of
Medical Research. |
Type of Sample
Upper respiratory specimens: nasopharyngeal and oropharyngeal
swabs; both swabs are placed together in a viral transport
medium and transported to the laboratory in ice.
Lower
respiratory specimens: sputum and/or endotracheal aspirate or
bronchoalveolar lavage in patients with more severe respiratory
disease (obtained with aerosol precautions)
Confirmatory Tests
(a) Respiratory tract or
blood samples tested positive for 2019-nCoV nucleic acid using
Real-time Reverse Transcriptase – Polymerase Chain Reaction
(RT-PCR)
(b) Genetic sequencing of respiratory tract or
blood samples is highly homologous with the known 2019-nCoV, but
this is not done routinely.
Serological tests may help
in epidemiological investigation but there could be cross
reactivity with other coronaviruses. Viral isolation is not done
routinely for diagnosis. Rapid diagnostic test kits like Xpert
Xpress SARS-CoV-2 by Cepheid has been approved by the US- FDA
(United States Food and Drug Administration) for Emergency Use
Authorization (EUA) and RealStar SARS-CoV-2 RT-PCR kit 1.0 by
Altona Diagnostics and Patho Detect by MY LAB have been approved
by ICMR[20,21].
Ancillary Investigations
Complete blood count: Lymphopenia was seen in 85% of
critically ill adults, suggesting it a marker of severe disease
while among the overall pediatric cases, it was seen in 3.5%
[7,12].
Infection markers: Elevation of C-reactive
protein (CRP) was reported in 20% and procalcitonin in 64% of
cases [7].
Radiological findings: Chest radiography (CXR)
or computed tomography (CT) are not recommended as a routine for
children but only in specific cases presenting with pneumonia
and/or acute respiratory distress syndrome (ARDS). Parenchymal
abnormalities with peripheral consolidations on CXR have been
reported in a small case series from Korea [14]. Ground glass
opacities (32%), local patchy shadows (18%) and bilateral patchy
shadows (12%) on CT chest were the common findings in children
[7]. Bilateral pneumonia (75%), unilateral pneumonia (25%) and
multiple mottling and ground-glass opacity (14%) were reported
based on CXR and CT findings from adult patients in Wuhan, China
[15].
Laboratory markers of organ dysfunction: Elevation
of transaminases is seen in 12-14% and d-Dimer in 14% cases [7].
PREPAREDNESS AND ADMINISTRATIVE CONCERNS FOR ICU
A phased and tiered plan for ICU during the
pandemic needs to be made based on the assessment of healthcare
burden and resource utilization [13,22,23].
Intensive
care units: Create cohort intensive care units where critically
ill confirmed COVID-19 patients will be managed. This would be a
different area from where other PICU patients are being managed
in order to reduce transmission within the hospital. In
addition, a separate area should be developed where suspected
COVID-19 patients will be managed. With increasing burden of
patients, general beds may have to be converted to ICU beds and
provided with suitable infrastructure. Predictive models based
on local epidemic need to be developed for expected number of
patients as well as need of equipment.
Setting up of
isolation rooms : Negative pressure isolation is the standard
recommendation for management of a suspected or proven COVID-19
patient. However, in case of non-availability of these rooms,
use single rooms with separate air outlet/exhaust, preferably on
the higher floor of the building. These rooms should be equipped
with resuscitation trolley, essential drugs, multipara monitor
and ventilator. Positive pressure rooms like operation theatres
are not suitable for airway management as aerosol generation is
higher.
Reducing the ICU burden: All elective non-urgent
admissions and surgeries need to be halted during the outbreak
in order to rationalize resource-utilization, and ensure
adequate back-up to handle the crisis.
Re-allocation of
staff: During the crisis, there may be acute shortage of
critical care specialists and nursing staff. It is essential to
identify staff from respiratory medicine, infectious disease and
other units who may be trained in infection control, personal
protective equipment (PPE) use and management of critically ill
patients.
Rotation of staff and reserve for back-up:
Adequate reserve of healthcare providers needs to be ensured as
a back-up in case of emergencies or healthcare professionals
falling sick. The team members should be working on rotation (in
a shift of 4-7 days) with adequate rest in between.
Training of all staff: All those who are likely to come in close
contact with the patient or are handling equipment,
surroundings, and waste management should receive training
regarding infection control including correct technique of
donning and doffing of PPE and disinfection of surfaces and
equipments. Proper training and a written plan (Standard
Operating Procedure) should be there for waste disposal.
Rational use of PPE: In view of current global shortage, WHO has
formulated guidelines for the rational use of PPE. This includes
co-ordination of PPE supply chain management mechanism,
appropriate PPE use based on indication, minimizing the need of
PPE by bundling activities, using physical barriers and
telemedicine where appropriate, and restricting visitors [24].
MANAGEMENT IN RESOURCE-LIMITED SETTINGS
Triage andTransport
A dedicated area for
screening and triaging of patients with suspected COVID-19 is
essential. Once the patient fits to the case definition and
requires admission, unnecessary movement must be avoided and
minimum staff should accompany the patient. Ensure that the
patient (if self-breathing) and the accompanying persons should
be on a 3-ply surgical mask.
ICU Management
Severe and critical cases need ICU care for monitoring,
ventilation and organ support therapy.
Severe acute
respiratory illness (SARI): SARI is defined by the presence of
cough and fast breathing plus at least one of the following
[25]:(i) Oxygen saturation (SpO2) <90%, (ii) severe chest
indrawing and grunting, and (iii) altered mental status.
SARI is the most common indication for ICU transfer and most
guidelines are similar to management of any viral pneumonia with
ARDS with an emphasis on minimizing risk of transmission to
others, especially healthcare workers [26,27]. The details on
the management of SARI are given in Part II of this write-up and
Table I.
Table I Treatment Based on Severity of Disease in Proven Coronavirus Disease-19 (COVID-19)
|
Symptomatic
proven case |
Admit in |
Treatment |
Discharge | |
Mild |
Designated COVID |
Symptomatic treatment |
Discharge if 72 h afebrile or 7d after symptom | |
isolation room | |
onset and two samples negative 24 h apart | |
|
| |
followed by home quarantine for total 14 d | |
Moderate |
Designated COVID |
Supportive care, oxygen |
Clinical improvement and two negative | |
isolation room |
Oseltamivir |
nCoV PCR tests 24 h apart | |
Severe |
COVID ICU |
Provide nasal prong oxygen |
Clinical improvement and two negative | | |
Escalate to invasive ventilation |
nCoV PCR tests 24 h apart | | |
if worsening | | | |
Avoid HFNC/NIV | | | |
Oseltamivir Ritonavir/Lopinavir | | | |
OR Hydroxychloroquine | | | |
Supportive care |
| |
Critical |
COVID ICU |
In addition to the above: |
Clinical improvement and two negative | | |
Intubate based on clinical/blood |
nCoV PCR tests 24 h apart | | |
gas/radiological features | | | |
Use all airborne precautions | | | |
Ventilation ARDS protocol | | | |
Other organ support | | | |
Once improving, wean from | | | |
ventilator and extubate as per protocol | | |
HFNC: High-flow nasal cannula, NIV: Non-invasive ventilation, ICU: intensive care unit,ARDS: Acute respiratory distress syndrome. |
Septic shock: Management of septic
shock in COVID is not very different from the routine. However,
the Surviving Sepsis Campaign (SSC) guidelines for COVID-19
recommend conservative fluid strategy, avoiding colloids as
resuscitation fluid, and to use low dose steroids in
catecholamine refractory shock [28]. In children, epinephrine is
the first vasoactive of choice for septic shock.
Co-infections: Co-infections like secondary bacterial pneumonia
are common, especially in children (50%) and addition of broad
spectrum antibiotic to cover gram positive, gram negative, and
staphylococcal infection is recommended [29].
Myocarditis: Cardiogenic shock with elevations in hypersensitive
Tropnonin-I have been seen in 12% of patients. Management
includes inodilators like milrin-one, diuretics,
immunomodulators (methylprednisolone and IVIG) and circulatory
support with ECMO (extra-corporeal membrane oxygenation) have
also been used in a few cases [30,31].
Acute kidney
injury : This has been reported in 7% and renal replacement
therapy may be necessary [32].
Supportive care: This
includes conservative fluid management, nutrition, appropriate
sedo-analgesia, and prevention and treatment of healthcare
associated infections.
Specific Therapy
Although no definitive therapy till date has proven benefit
for SARS-CoV2, antiviral drugs like Remdesivir,
Lopinavir/Ritonavir are being used in over 50% of the critically
ill adults based on in vitro viral inhibition and recovery in
SARS and MERS but there is no strong evidence [33–36].
Chloroquine has been found to increase endosomal pH and hinder
virus cell fusion and also interfere with ACE2, a receptor for
binding of SARS-CoV2 [37]. A combination of hydroxychloroquine
and azithromycin showed reduction in viral load [38].
Interferons, IVIG, and convalescent plasma from recovered SARS
patients are other tested treatment options [39]. Vaccination
for RNA viruses (measles, influenza, polio) has shown higher
titers of neutralizing antibodies against SARS-CoV [40]
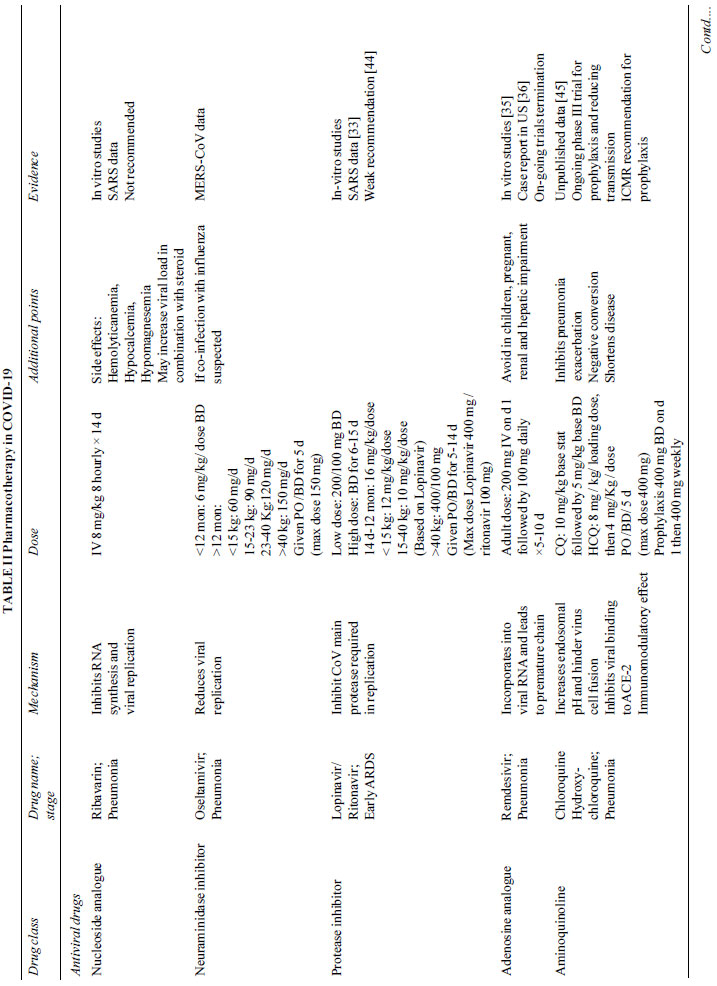
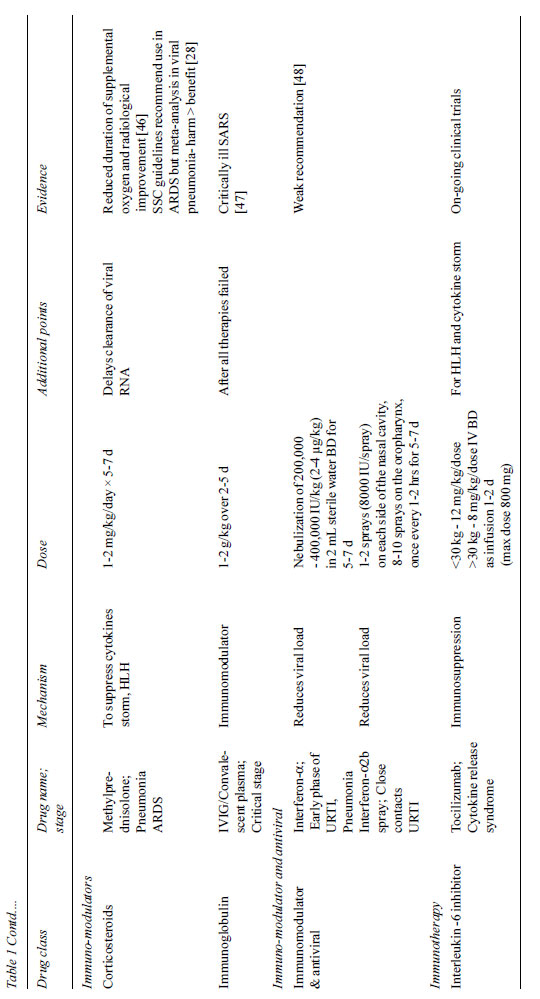
(Table II). Based on the current experience, we may use
broad spectrum antibiotics, oseltamivir, protease inhibitors,
hydro-xychloroquine and azithromycin. Lopinavir/Ritonavir along
with Chloroquine should be avoided in combination.
 |
 |
Course and Recovery
In adult patients with COVID-19 pneumonia, onset of symptoms
to respiratory failure takes an average of 7 days with peak
severity at 10 days. Signs of improvement starts occurring by
day 14. However, at the time of reporting of most studies, many
patients were still admitted and their course needs to be
followed to know the exact prognosis [40].
INFECTION PREVENTION AND CONTROL
In the
intensive care setting, disinfection of high–touch surfaces like
monitors, ventilator screen, other equipment, resuscitation
trolleys etc are essential and need to be carried out every 4
hours.
Surface decontamination: Alcohol (e.g. isopropyl
70% or ethyl alcohol 70%) can be used to wipe down surfaces
where the use of bleach is not suitable for e.g. Mobiles,
laptops, keys, pens etc.
Disinfection: Freshly prepared1%
sodium hypochlorite should be used as a disinfectant for
cleaning and disinfection with at least 10 minute contact
period.
Aerosol: Ensure room disinfection within 20
minutes of any procedure generating aerosol.
Social
distancing: Maintain at least 1 meter distance unless required
for examination or procedure.
Contact and droplet
precautions: minimize direct contact, ensure hand hygiene, and
cough etiquette.
Healthcare Worker (HCW) Risks
Apart from risks related to droplet spread and from
contaminated surfaces, ICU professionals face the challenge of
acquiring infection during aerosol generating procedures (see
table in Part II). HCW should wear a medical mask and gown when
entering a room where patients with suspected or confirmed
COVID-19 are admitted and use full personal protective equipment
(PPE), which includes N95 mask, goggles or face shield, cap,
full sleeve gown and shoe cover, when performing
aerosol-generating procedures [41]. The entire PPE is
recommended to be used for 4-6 hours and changed earlier if
there is any soiling. Team should not include staff vulnerable
to infection like immunocompromised person, pregnant ladies, age
>60 years or those with co-morbidities. In the event of exposure
and manifestation of infection, management as per guidelines as
well as psychosocial support needs to be ensured. Adequate
communication, education and adherence to strict personal
protection can minimize the risk of transmission to HCW [26].
ICMR recommends prophylactic use of hydroxychloroquine 400 mg
twice a day on day 1, followed by 400 mg once weekly for next 7
weeksfor HCW managing suspected or confirmed COVID-19 patients
[42].
Special Considerations for Resuscitation
It is important to minimize the number of
people inside the room during high aerosol generating events
like cardiopulmonary resuscitation. One airway specialist, one
nurse/doctor for chest compression and one nurse for medication
are essential. Other assistants may remain outside the room and
may enter only if necessary after donning full PPE. Hand bagging
needs to be avoided. During any disconnection from ventilator,
endotracheal (ET) tube needs to be clamped and/or viral filter
attached to the ET tube. In case re-intubation is required,
follow the standard procedure described (see Part II in this
issue).
CONCLUSION
The COVID-19
pandemic caused by 2019-nCOV has become a serious concern for
mankind all over the world. It has challenged and overwhelmed
the existing intensive care facilities globally. SARI is the
most common indication for intensive care management and is
associated with high mortality. The disease so far appears to be
less common in children and seems to have a milder course.
Preparation for handling crisis during this outbreak is
essential for early identification, stratification and
management of cases. Prevention by ensuring strict infection
control practices minimizes transmission to other patients and
healthcare workers, especially in intensive care units.
Contributors: NR, KN, AB, SKA: substantial contribution to the
conception and design of the work (ii) drafting the work (iii)
final approval of the version to be published (iv) agreement to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved; GVB, MS,
RL, DG, MJ: substantial contributions to the acquisition and
interpretation of data for the work (ii) revising it critically
for important intellectual content (iii) Final approval of the
version to be published (iv) Agreement to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Funding: None; Competing
interests: None stated.
REFERENCES
1. Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (covid-19) outbreak in
China: Summary of a report of 72/ 314 cases from the Chinese
center for disease control and prevention [published online
ahead of print]. JAMA. 2020;10.1001/jama.2020.2648. Available
from: https://jamanetwork.com/journals/jama/fullarticle/2762130.
Accessed March 25, 2020.
2. Shen K, Yang Y, Wang T, Zhao
D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention
of 2019 novel coronavirus infection in children: experts’
consensus statement. World J Pediatr (2020).
https://doi.org/10.1007/s12519-020-00343-7. Accessed March 25,
2020.
3. Coronavirus disease 2019 [Internet]. [cited 2020
Mar 26]. Available from:
https://www.who.int/emergencies/diseases/
novel-coronavirus-2019.
4. de Wilde AH, Snijder EJ,
Kikkert M, van Hemert MJ. Host factors in coronavirus
replication. Curr Topic Microbiol Immunol. 2018;419:1-42.
5. Paules CI, Marston HD, Fauci AS. Coronavirus
infections–More than just the common cold. JAMA. 2020;323:707–8.
6. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al.
Early transmission dynamics in Wuhan, China, of novel
coronavirus–infected pneumonia. N Engl J Med. 2020;
382:1199-207.
7. Lu X, Zhang L, Du H, Zhang J, Li Y, Qu
J, et al. SARS-CoV-2 Infection in Children. New England J Med.
2020. Available from: https://www.nejm.org/doi/
full/10.1056/NEJMc2005073. Accessed March 24, 2020.
8.
Lee P-I, Hu Y-L, Chen P-Y, Huang Y-C, Hsueh P-R. Are children
less susceptible to COVID-19? JMicrobiol Immunol Infect. 2020.
Available from:
https://www.sciencedirect.com/science/article/pii/S1684118220300396?via%3Dihub.
Accessed March 24, 2020.
9. Li W, Moore MJ, Vasilieva N
Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2
is a functional receptor for the SARS coronavirus. Nature.
2003;426:450-54.
10. Mahase E. Coronavirus: covid-19 has
killed more people than SARS and MERS combined, despite lower
case fatality rate. BMJ. 2020;368:m641. Available from
https://www.bmj.com/content/368/bmj.m641. Accessed March 29,
2020.
11. Onder G, Rezza G, Brusaferro S. Case-fatality
rate and characteristics of patients dying in relation to
COVID-19 in Italy. JAMA. Published online March 23, 2020.
doi:10.1001/jama.2020.4683. Accessed March 29, 2020.
12.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course
and outcomes of critically ill patients with SARS-CoV-2
pneumonia in Wuhan, China: A single-centered, retrospective,
observational study. Lancet Respiratory Medicine. 2020.
Available from: https://doi.org/10.1016/S2213-2600(20)30079-5.
Accessed March 29, 2020
13. Grasselli G, Pesenti A,
Cecconi M. Critical care utilization for the COVID-19 outbreak
in Lombardy, Italy: Early experience and forecast during an
emergency response. JAMA. Published online March 13, 2020.
doi:10.1001/jama.2020.4031. Accessed March 29, 2020
14.
Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest
radiographic and CT findings of the 2019 Novel Coronavirus
Disease (COVID-19): Analysis of nine patients treated in Korea.
Korean J Radiol. 2020;21:494-500.
15. Chen N, Zhou M,
Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia
in Wuhan, China: A descriptive study. Lancet. 2020;395:507-13.
16. Available from:
https://www.who.int/docs/default-source/coronaviruse/situation-reports-48.Accessed
March 29, 2020
17. Dong Y, Mo X, Hu Y, Qi X, Jiang F,
Jiang Z, et al. Epidemiological characteristics of 2143
pediatric patients with 2019 coronavirus disease in China.
Pediatrics. 2020; doi: 10.1542/peds.2020-0702. Accessed March
29, 2020
18. Indian Council of Medical Research. Revised
Strategy of COVID19 testing in India (Version 3, dated
20/03/2020). Available from:
https://icmr.nic.in/sites/default/files/
upload_documents/2020-03-20_covid19_test_v3.pdf. Accessed March
29, 2020
19. World Health
Organization. (2020). Laboratory testing for coronavirus disease
2019 (COVID-19) in suspected human cases: Interim guidance, 2
March 2020. World Health Organization. Available from:
https://apps.who.int/iris/handle/10665/331329. Accessed March
30, 2020.
20. Xpert® Xpress SARS-CoV-2. Instructions for
Use. Cepheid, California, USA; 2020. Available from:
https://www.fdagov/media/136314/download. Accessed March 30,
2020
21. Indian Council of Medical Research. Press
Release on “Fast Track Approval for Indian COVID-19 Testing Kits
for Commercial Use.” Available from:
https://www.icmr.nic.in/content/press-release-fast-track-approval-indian-covid-19-testing-kits-commercial-use.
Accessed March 30, 2020
22. The Australian and New
Zealand Intensive Care Society (ANZICS). COVID-19 Guidelines
Version 1. Available from:
http://cec.health.nsw.gov.au/__data/assets/pdf_file/0004/572512/
ANZICS-COVID-19-Guidelines-Version-1.pdf. Accessed March 30,
2020.
23. Xie J, Tong Z, Guan X, Du B, Qui H, Slutsky AS.
Critical care crisis and some recommendations during the
COVID-19 epidemic in China. Intensive Care Med. 2020. Available
from: https://doi.org/10.1007/s00134-020-05979-7. Accessed March
29, 2020.
24. Available from:
https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf.
Accessed March 29, 2020.
25. Clinical management of
severe acute respiratory infection (SARI) when COVID-19 disease
is suspected Interim guidance. WHO/2019-nCoV/clinical/2020.4.
Accessed March 29, 2020.
26. Murthy S, Gomersall CD,
Fowler RA. Care for Critically Ill Patients With COVID-19. JAMA.
Published online March 11, 2020. doi:10.1001/jama.2020.3633.
Accessed March 29, 2020.
27. Brewster DJ, Chrimes NC, Do
TBT, Fraser K, Groombridge CJ, Higgs A, et al. Consensus
statement: Safe Airway Society principles of airway management
and tracheal intubation specific to the COVID-19 adult patient
group. Med J Aust. March 16, 2020. Accessed March 29, 2020
28. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan
E, et al. Surviving Sepsis Campaign: Guidelines on the
Management of Critically Ill Adults with Coronavirus Disease
2019 (COVID-19). Intensive Care Med.
https://doi.org/10.1007/s00134-020-06022-5. Accessed March 29,
2020
29. Xia W,Shao J, Guo Y, Peng X, Li Z, Hu D.
Clinical and CT features in pediatric patients with COVID 19
infection: Different points from adults.
doi.org/10.1002/ppul.24718. Accessed March 29, 2020
30.
Hongde Hu, Fenglian Ma, Xin Wei, Yuan Fang. Coronavirus
fulminant myocarditis saved with glucocorticoid and human
immunoglobulin, Eur Heart J. 2020; ehaa190. https://
academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaa190/5807656.Accessed
March 29, 2020
31. Zeng J, Liu Y, Yuan J, Wang F, Wu W,
Li J, et al. First case of COVID-19 infection with fulminant
myocarditis complication: Case report and insights [Pre-print].
Preprints 2020, 2020030180. Available from
https://www.preprints.org/manuscript/202003.0180/v1.Accessed
March 29, 2020.
32. Huang C, Wang Y, Li X, Ren L, Zhao J,
Hu Y, et al. Clinical features of patients infected with 2019
novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
33. Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MML, et
al. Treatment of severe acute respiratory syndrome with
lopinavir/ritonavir: A multicentre retrospective matched cohort
study. Hong Kong Med J. 2003;9:399-406.
34. Cao B, Wang
Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of
lopinavir–ritonavir in adults hospitalized with severe Covid-19.
New Engl J Med. 2020 [Online early]. Available from:
https://www.nejm.org/doi/full/10.1056/NEJMoa2001282.Accessed
March 29, 2020
35. Wang M, Cao R, Zhang L, Yang X, Liu J,
Xu M, et al. Remdesivir and chloroquine effectively inhibit the
recently emerged novel coronavirus (2019-nCoV) in vitro. Cell
Res 2020;30:269-71.
36. Holshue ML, DeBolt C, Lindquist
S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel
coronavirus in the United States. N Engl J Med. 2020;382:929-36.
37. Vincent M, Bergeron E, Benjannet S, Erickson B,
Rollin P, Ksiazek T, et al. Chloroquine is a potent inhibitor of
SARS coronavirus infection and spread. Virol J. 2005;2:69.
38. Gautret P, Lagiera J, Parolaa P, Hoanga V, Meddeba L,
Mailhe M, et al. Hydroxychloroquine and azithromycin as a
treatment of COVID 19: Results of an open label non randomized
clinical trial. Int J Antimicrob Agent. 2020 [Online early]
Available from:
http://www.sciencedirect.com/science/article/pii/S0924857920300996.
Accessed March 29, 2020.
39. Wang BX, Fish EN. Global
virus outbreaks: interferons as 1st responders. SeminImmunol.
2019;43:101300. Available from
http://www.sciencedirect.com/science/article/pii/S1044532319300065.Accessed
March 29, 2020.
40. Zhang L, Liu Y. Potential
interventions for novel corona-virus in China: A systematic
review. J Med Virol. 2020;92:479-90.
41. Pan F, Ye T,
Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes
on chest ct during recovery from 2019 novel coronavirus
(COVID-19) pneumonia. Available
from:https://pubs.rsna.org/doi/10.1148/radiol. 2020200370.
Accessed March 29, 2020.
42. World Health Organization.
Advice on the use of masks in the community, during home care
and in healthcare settings in the context of the novel
coronavirus (COVID-19) outbreak [internet]. Available from:
https://www.who.int/publications-detail/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak.
Accessed March 25, 2020.
43. Indian Council of Medical
research. Recommendation for empiric use of hydroxy-chloroquine
for prophylaxis of SARS-CoV-2 infection [internet]. Available
from:
https://icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommendation_22March_final_MM.pdf.Accessed
March 25, 2020.
44. Wang XF, Yuan J, Zheng YJ, Chen J,
Bao YM, Wang YR, et al. Clinical and epidemiological
characteristics of 34 children with 2019 novel coronavirus
infection in Shenzhen [English abstract]. Zhonghua Er Ke Za Zhi.
2020;58:E008. [Retracted].
45. Gao J, Tian Z, Yang X.
Breakthrough: Chloroquine phosphate has shown apparent efficacy
in treatment of COVID-19 associated pneumonia in clinical
studies. Biosci Trends. 2020;14:72-3.
46. Wang Y, Jiang
W, He Q, Wang C, Wang B, Zhou P, et al. Early, low-dose and
short-term application of corticosteroid treatment in patients
with severe COVID-19 pneumonia: single-center experience from
Wuhan, China [pre-print]. Available from:
https://doi.org/10.110/2020.03.06.20032342. Accessed March 29,
2020.
47. Chen L, Xiong J, Bao L, Shi Y. Convalescent
plasma as a potential therapy for COVID-19[Published online
ahead of print]. Lancet Infect Dis. 2020;S1473-3099(20)30141-9.
Available from: https://doi.org/10.1016/S1473-3099(20)
30141-9.Accessed March 29, 2020.
48. Jin Y, Cai L, Cheng
Z, Cheng H, Deng T, Fan Y, et al. A rapid advice guideline for
the diagnosis and treatment of 2019 novel coronavirus
(2019-nCoV) infected pneumonia (standard version). Military Med
Res. 2020;7: 4. Available from: https://mmrjournal.
biomedcentral.com/articles/10.1186/s40779-020-0233-6. Accessed
March 29, 2020.
|
|
|
 |
|

