|
|
|
Indian Pediatr 2019;56:294-298 |
 |
Early Aggressive
Enteral Feeding in Neonates Weighing 750-1250
Grams: A Randomized Controlled Trial
|
|
Manoj Modi, Siddarth Ramji, Ashish Jain, Pradeep
Kumar and Neeraj Gupta
From Department of Pediatrics, Maulana Azad medical
college, New Delhi, India.
Correspondence to: Dr Manoj Modi, Department of
Neonatology, Sir Ganga Ram hospital, New Delhi, India.
[email protected]
Received: November 17, 2017;
Initial review: March 31, 2018;
Accepted: February 21, 2019.
Clinical Trial
Registration: CTRI/2014/06/004663.
|
Background: In preterm neonates, enteral feeding
is advanced slowly, considering the risk of necrotizing enterocolitis.
Prolonged intravenous alimentation in these neonates, however, may
increase the risk of sepsis-related morbidity and mortality,
particularly in low resource settings.
Objectives: Objective of this was study to
evaluate impact of aggressive enteral feeding on mortality and
morbidities among preterm neonates.
Design: Randomized controlled trial.
Participants: Neonates with birthweight 750-1250
g.
Interventions: 131 preterm neonates with
birth weight 750-1250 g, admitted to neonatal intensive care unit
between April 2012 and June 2014, were randomized to aggressive feeding
or conservative feeding regimen.
Outcomes: The primary outcome of the study was
all-cause mortality during hospital stay. The secondary outcomes
included proportion of sepsis (blood culture proven), necrotizing
enterocolitis, feed intolerance, survival without major morbidity at
discharge, time to reach full enteral feed (180 mL/kg/d), duration of
hospitalization, and average daily weight gain (g/kg).
Results: All-cause mortality was 33.3% in
aggressive regimen and 43.1% in conservative regimen, [RR (95%) CI 0.77
(0.49, 1.20)]. Neonates with aggressive feeding regimen reached full
enteral feed earlier; median (IQR) 7 (6, 8) days compared to
conservative regimen, 10 (9, 14) days; P <0.001. There was no
difference in culture positive sepsis rate, survival without major
morbidities, feed intolerance, necrotizing enterocolitis, duration of
hospitalization and average daily weight gain.
Conclusions: In neonates with birth weight
750-1250 g, early aggressive feeding regimen is feasible but not
associated with significant reduction in all-cause mortality, culture
positive sepsis or survival without major morbidities during hospital
stay. Neonates with aggressive regimen have fewer days on IV fluids and
reach full feed earlier.
Keywords: Enteral feeding, Morbidity,
Mortality, Necrotizing enterocolitis, Prematurity, Sepsis.
|
|
A
ggressive enteral feeding has been considered a
potential risk factor for necrotizing enterocolitis (NEC) in very low
birth weight (VLBW) neonates [1-3].
This has often delayed the introduction of enteral feeds
in very preterm infants and infants weighing less than 1250 grams. The
subsequent grading up of enteral feed volumes has been slow, with
parenteral nutrition bridging the nutritional gap till achievement of
full enteral feeding. The evidence to support this practice is
inadequate and weak. On the contrary, this practice could diminish the
functional adaptation of the preterm gastrointestinal tract [4,5].
Prolonged exposure to parenteral nutrition may
increase the risk of metabolic complications, bloodstream infections and
mortality during neonatal intensive care unit (NICU) stay, and poor
subsequent growth and neurodevelopmental outcome, especially in low- and
middle-income countries [6-10].
The present study was designed to test the hypothesis
whether an early aggressive feeding regimen in preterm infants with
birth weight 750-250 g would result in a lower mortality and morbidity
compared to conservative feeding regimen.
Methods
This randomized controlled open label trial was
conducted at a medical school affiliated hospital between April 2012 and
June 2014. All inborn neonates with a birthweight of 750-1250 g were
screened for eligibility. Neonates with gross congenital malformation of
the gastrointestinal tract, severe birth asphyxia (Apgar score <3 at 1
min), and those who could not be fed by enteral route for first four
days of life were excluded. The protocol of the study was approved by
the institutional ethics committee.
The primary outcome of the study was all-cause
mortality during hospital stay. The secondary outcomes included sepsis
(blood culture proven), necrotizing enterocolitis (NEC) stage II or
more, feed intolerance (presence of one or more of following:
distended/tense/tender abdomen, increase in abdominal girth by >2 cm in
a 2-hour interval, hemorrhagic/bilious aspirate), survival without major
morbidity (bronchopulmonary dysplasia/intraventricular hemorrhage grade
III or IV/cystic periventricular leucomalacia/ retinopathy of
prematurity requiring treatment) at discharge, time to reach full
enteral feed (180 mL/kg/d) and average daily weight gain (g/kg/d) during
NICU stay, after achieving birthweight. NEC was defined as per modified
Bell’s staging [11]; bronchopulmonary dysplasia (BPD) was defined as per
National Institute of Health consensus definition 2001 [12];
intraventricular hemorrhage (IVH) was defined as per Papile’s
classification [13]; and periventricular leucomalacia (PVL) was defined
as per de Vries classification [14].
Eligible neonates were enrolled after informed
written consent of parents. Neonates were randomly assigned to early
aggressive feeding regimen (AR) or conservative feeding regimen (CR)
using computer generated block randomization sequence of variable block
sizes (4, 6 or 8) stratified for birth weight (750-1000 g and 1001-1250
g). Allocation concealment was ensured by placing the sequence in sealed
opaque envelopes. Blinding was not possible due to the nature of the
intervention,
Neonates were considered eligible for initiating
enteral feeds if they were not on any ionotrope support; and if on
mechanical ventilation, had a Mean Airway Pressure <14 mbar and/or
Fraction inspired Oxygen <0.7; and the abdomen was soft. In the
conservative regimen (CR) group neonates with birthweight 750-1000 g
were initiated at a feed volume of 15 mL/kg/day, with subsequent
advancement by 15 mL/kg/day. Neonates with birth weight 1001-1250 g were
initiated at a volume of 20 mL/kg/day feed, with subsequent daily
increments of 20 mL/kg/day. In the aggressive regimen (AR), neonates
with birthweight 750-1000 g were initiated with 30 mL/kg/day feeds with
subsequent increments of 30 mL/kg/day. In neonates with birthweight
1001-1250 g, feed was initiated at 40 mL/kg/day with subsequent daily
increments of 40 mL/kg/day. In both treatment arms, feeds were given as
2-hourly interval bolus feeds, and increments maximized upto 180 mL/kg/day.
Mother’s own milk was preferred, whenever available. If mother’s milk
was not available, preterm formula was used. To meet the fluid and
nutritional needs not met by enteral feeds, neonates also received
parenteral nutrition till the infant tolerated 100-120 mL/kg/day of
enteral feeds.
In all enrolled neonates, baseline maternal,
antenatal, intrapartum and neonatal details were recorded. Neonates were
monitored for feed intolerance and NEC. If abdomen girth increased by >2
cm between feeds or abdomen was tense/tender, gastric aspiration was
done to assess type and volume of gastric residues. If abdominal girth
had increased by >2 cm and/or residual feed volume was >50% of previous
feed volume, feed was withheld for 24 hours or till abdominal signs
resolved, whichever was later. If residual feed was 20-50% of feed
volume, feeding was continued without an increment for next 24 hrs; if
residual volume was <20% of feed volume, increments were made as per
assigned group protocol. Enteral feed was also withheld, if neonate was
receiving inotropes. In neonates with suspected sepsis, culture data
were also recorded.
Sample size was calculated based on our pilot
observation, where with conservative feeding, mortality among neonates
with birthweight <1250 g was 80%. To detect an expected
30% relative reduction in mortality with the aggressive feeding, 58
neonates were required in each arm for a power of 80% and alpha error of
0.05. We planned to recruit 65 neonates in each arm, expecting 10%
attrition of participants.
Statistical analysis: Categorical data were
compared using Chi-square test. Continuous data were compared using
student t-test or Mann-Whitney U-test. Time to event outcomes were
analyzed using Kaplan-Meier curve. A P value of <0.05 was
considered as significant.
Results
Fig. 1 depicts the study flow. We enrolled 66
neonates in aggressive feeding, and 65 in the conservative feeding
group. Table I provides a comparison of baseline
characteristics between the study groups. Primary and secondary outcomes
of study are depicted in Table II.
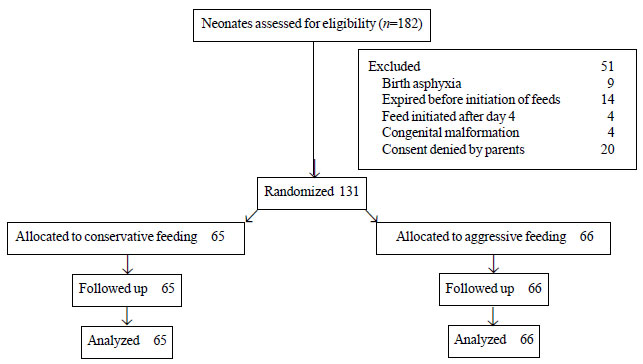 |
|
Fig. 1 Flow of participants in the
study.
|
TABLE I Baseline Characteristics of Enrolled Neonates
|
Variables |
Aggressive |
Conservative |
|
regimen |
regimen |
|
(n=66) |
(n=65) |
|
Birthweight (g), mean (SD) |
1085 (112) |
1067 (144) |
|
Gestation (wk), mean (SD) |
31.3 (2.8) |
30.6 (2.2) |
|
Fetal growth restriction, n (%) |
41 (62.1) |
36 (55.3) |
|
Male gender, n (%) |
32 (48) |
37 (56) |
|
Gestational hypertension, n (%) |
16 (24.2) |
18 (27.6) |
|
#Absent/reversed flow, n (%) |
13(19.6) |
16 (24.6) |
|
Antenatal steroid received, n (%) |
56 (84.8) |
51 (78.4) |
|
Cesarean section, n (%) |
13 (19.7) |
12 (18.5) |
|
Need for resuscitation at birth, n (%) |
17 (25.8) |
11 (16.9) |
|
Respiratory distress at birth, n (%) |
19 (28.7) |
26 (40) |
|
*Age enteral feed initiated (d) |
2 (1, 2) |
2 (1, 2) |
|
*median (IQR); #End-diastolic flow in umbilical artery. |
TABLE II Comparison of Primary and Secondary Outcomes in Two Feeding Groups
|
Outcome variables |
Aggressive |
Conservative |
RR (95% CI) |
P value |
|
regimen (n=66) |
regimen (n=65) |
|
|
|
Mortality, n (%) |
22 (33.3) |
28 (43.1) |
0.77 (0.49-1.20) |
0.25 |
|
Sepsis, n (%) |
17 (25.8) |
24 (36.9) |
0.69 (0.41-1.17) |
0.17 |
|
Feed intolerance, n (%) |
12 (18.2) |
17 (26.2) |
0.69 (0.36-1.33) |
0.27 |
|
NEC stage II/III, n (%) |
1 (1.5) |
2 (3) |
0.49 (0.04-5.29) |
0.55 |
|
IVH grade III,IV/cystic PVL, n (%) |
3 (4.5) |
4 (6.1) |
0.73 (0.17-3.17) |
0.68 |
|
Survival without major morbidities, n (%) |
43 (65.2) |
34 (52.3) |
1.2 (0.93-1.66) |
0.14 |
|
Time to reach full feed (d), median (IQR) |
7 (6, 8) |
10 (9, 14) |
- |
<0.01 |
|
Duration of hospital stay (d), median (IQR) |
24.9 (21.6, 28.2) |
26.6 (22.8, 30.3) |
- |
0.68 |
|
Average daily weight gain (g/kg), mean (SD) |
12.2 (5.4) |
11.7 (4.4) |
- |
0.66 |
There was a trend towards lower all-cause mortality
in the aggressive feeding group, but it was not statistically
significant (P=0.25). Frequency of sepsis, NEC and feed
intolerance, duration of hospital stay, and weight gain were also
comparable between the groups. Neonates in aggressive regimen had fewer
days on intravenous fluids and reached full feed earlier (P<0.001).
Discussion
In the present study, aggressive enteral feeding was
not associated with a significant reduction in mortality or other
morbidities. Neonates in aggressive feeding regimen had a reduced
duration of intravenous alimentation and reached full enteral feeds
earlier. Incidence of NEC and feed intolerance was comparable in two
regimens.
Findings of our study are consistent with most
previous observations, where neonates in rapid advancement group
achieved full feeds earlier and had significantly fewer days of
intravenous fluids, regained birthweight earlier and had shorter length
of stay in hospital, with a comparable mortality and morbidity [15-22].
In a recent randomized controlled trial, Sanghvi, et al. [23]
assessed feasibility of exclusive enteral feeding without any parenteral
nutrition in neonates with birthweight 1200-1500 g. They observed that
exclusive enteral feeding in this population was feasible with shorter
duration of hospital stay.
The strength of our study was enrolment of relatively
smaller neonates, who are more at risk of NEC and other morbidities.
Abnormal umbilical artery doppler and need for ventilatory requirement
were not excluded from the study, making our findings more generalizable
to sick and growth-restricted neonates. Limitations of our study were
inability to mask intervention from caregivers and investigator, and
inadequate power for primary outcome.
Findings of our study reaffirm the feasibility of
early aggressive feeding regimen in neonates, 750-1250 g birth weight.
No significant difference in mortality in our study could be due to fact
that we calculated sample size for hypothesized 30% relative reduction
with aggressive feeding with an estimated 80% mortality with
conventional feeding. However, mortality during study period with
conventional feeding regimen was 43%. A 10% absolute reduction in
mortality did not reach statistical significance, perhaps due to
inadequate power of study. On post-hoc calculation, power of our study
to detect this difference was 32.5%. To detect this observed different
with 80% power, 367 neonates in each group would be required. Similarly,
due to small sample size, we could not substantiate difference in
culture-positive sepsis or survival without major morbidities.
We conclude that early aggressive feeding regimen in
neonates with birth weight 750-1250 g does not seem to be associated
with significant reduction in all-cause mortality, culture positive
sepsis or survival without major morbidities during NICU stay.
Aggressive feeding regimen seems to be well tolerated in this population
with reduction in duration of intravenous fluids with early achievement
of full enteral feeding.
|
What is Already Known?
•
Early aggressive feeding in VLBW neonates is considered to
increase the risk of feed intolerance and necrotizing
enterocolitis.
What This Study Adds?
•
Aggressive enteral feeding in neonates with birthweight
750-1250 g does not seem to affect mortality or risk of feed
intolerance.
|
References
1. Anderson DM, Kliegman RM. The relationship of
neonatal alimentation practices to the occurrence of endemic necrotizing
enterocolitis. Am J Perinatol. 1991;8:62-7.
2. Brown EG, Sweet AY. Preventing necrotizing
enterocolitis in neonates. JAMA. 1978;240:2452-4.
3. McKeown RE, Marsh TD, Amarnath U, Garrison CZ,
Addy CL, Thompson SJ, et al. Role of delayed feeding and of
feeding increments in necrotizing enterocolitis. J Pediatr.
1992;121:764-70.
4. Berseth CL. Neonatal small intestinal motility:
motor responses to feeding in term and preterm infants. J Pediatr.
1990;117:777-82.
5. Burrin DG, Stoll B. Key nutrients and growth
factors for the neonatal gastrointestinal tract. Clin Perinatol.
2002;29:65-96.
6. Hartel C, Haase B, Browning-Carmo K, Gebauer C,
Kattner E, Kribs A, et al. Does the enteral feeding advancement
affect short-term outcomes in very low birth weight infants? J Pediatr
Gastroenterol Nutr. 2009;48:464-70.
7. Morris BH, Miller-Loncar CL, Landry SH, Smith KE,
Swank PR, Denson SE. Feeding, medical factors, and developmental outcome
in premature infants. Clin Pediatr (Phila). 1999;38:451-7.
8. Basu S, Rathore P, Bhatia BD. Predictors of
mortality in very low birth weight neonates in India. Singapore Med J.
2008;49:556-60.
9. Narayan S, Aggarwal R, Upadhyay A, Deorari AK,
Singh M, Paul VK. Survival and morbidity in extremely low birth weight
(ELBW) infants. Indian Pediatr. 2003;40:130-5.
10. Mukhopadhyay K, Louis D, Mahajan R, Kumar P.
Predictors of mortality and major morbidities in extremely low birth
weight neonates. Indian Pediatr. 2013;50: 1119-23.
11. Kliegman RM, Walsh MC. Neonatal necrotizing
enterocolitis: pathogenesis, classification, and spectrum of illness.
Curr Probl Pediatr. 1987;17:213-88.
12. Jobe AH, Bancalari E. Bronchopulmonary dysplasia.
Am J Respir Crit Care Med. 2001;163:1723-9.
13. Papile LA, Burstein J, Burstein R, Koffler H.
Incidence and evolution of subependymal and intraventricular hemorr-hage:
a study of infants with birth weights less than 1,500 gm. J Pediatr.
1978;92:529-34.
14. de Vries LS, Eken P, Dubowitz LM. The spectrum of
leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1-6.
15. Rayyis SF, Ambalavanan N, Wright L, Carlo WA.
Randomized trial of "slow" versus "fast" feed advancements on the
incidence of necrotizing enterocolitis in very low birth weight infants.
J Pediatr. 1999;134:293-7.
16. Caple J, Armentrout D, Huseby V, Halbardier B,
Garcia J, Sparks JW, et al. Randomized, controlled trial of slow
versus rapid feeding volume advancement in preterm infants. Pediatrics.
2004;114:1597-600.
17. Salhotra A, Ramji S. Slow versus fast enteral
feed advancement in very low birth weight infants: a randomized control
trial. Indian Pediatr. 2004;41:435-41.
18. Krishnamurthy S, Gupta P, Debnath S, Gomber S.
Slow versus rapid enteral feeding advancement in preterm newborn infants
1000-1499 g: a randomized controlled trial. Acta Paediatr. 2010;99:42-6.
19. Karagol BS, Zenciroglu A, Okumus N, Polin RA.
Randomized controlled trial of slow versus rapid enteral feeding
advancements on the clinical outcomes of preterm infants with 750-1250g.
J Parenter Enteral Nutr. 2013;37:223-8.
20. Jain S, Mukhopadhyay K, Jain V, Kumar P. Slow
versus rapid enteral feed in preterm neonates with antenatal absent end
diastolic flow. J Matern Fetal Neonatal Med. 2016;29:2828-33.
21. Morgan J, Young L, McGuire W. Delayed
introduction of progressive enteral feeds to prevent necrotising
enterocolitis in very low birth weight infants. Cochrane Database Syst
Rev. 2014;12:CD001970.
22. Morgan J, Young L, McGuire W. Slow advancement of
enteral feed volumes to prevent necrotising enterocolitis in very low
birth weight infants. Cochrane Database Syst Rev. 2014;12:CD001241.
23. Sanghvi KP, Joshi P, Nabi F, Kabra N. Feasibility
of exclusive enteral feeds from birth in VLBW infants >1200 g – An RCT.
Acta Paediatr. 2013;102:e299-304.
|
|
|
 |
|

