|
|
|
Indian Pediatr 2017;54: 295-297 |
 |
Outcome of Antenatally
Presenting Posterior Urethral Valves (PUV) in Children
|
|
TP Joseph, VK Gopi, PR Babu and KV Satish Kumar
From Department of Pediatric Surgery, Baby Memorial
Hospital Ltd, Indira Gandhi Road, Calicut, Kerala, India.
Correspondence to: Dr Satish Kumar KV, 26/194 A, Sai
Sannidhi, Vadakkathparamba, Govindapuram (PO), Calicut 673 016, Kerala,
India.
Email: [email protected]
Received: February 25, 2016;
Initial review: May 19, 2016;
Accepted: November 30, 2016.
Published online: December 05, 2016.
PII:S097475591600030
|
Objective: To analyze the outcome of children with posterior
urethral valves who presented with antenatal hydronephrosis.
Methods: A 10-year retrospective
review of records of 70 children with posterior urethral valves.
Results: The mean (SD)
gestational age at diagnosis was 34 (4.48) weeks, and age at
intervention was 130.5 (170.9) days. The nadir creatinine was
significantly raised (>1.2 mg/dl) in children with oligohydramnios and
diversion.
Conclusion: All boys with
antenatally detected hydronephrosis need postnatal evaluation to rule
out posterior urethral valves. Short term outcome is improved with
postnatal treatments, and longer follow-up is needed to ensure a
favourable outcome.
Keywords: Antenatal diagnosis,
Hydronephrosis, Outcome, Ultrasound.
|
|
P
osterior urethral valves (PUV) are the most
common cause of obstructive uropathy [1,2],
and 25-30% of treated patients are at risk of developing
End-stage renal disease (ESRD) [3,4]. Sixty percent of renal transplants
in children are done for obstructive uropathy [5]. Routine use of
antenatal ultrasound has led to posterior urethral valves (PUV) being
increasingly diagnosed antenatally. Antenatal interventions are being
carried out in specialized centers to improve postnatal outcome [6]. In
centers without these facilities, better postnatal outcomes can be
achieved with early treatment, prevention of infection, and long-term
care.
Methods
We conducted a 10-year (2005-2015) retrospective
review of all operated cases of PUV, who had antenatal hydronephrosis.
Antenatal ultrasound findings, clinical features, age at confirmation of
diagnosis, biochemical abnormalities, surgical management and follow-up
data were analyzed. Initial investigations included urinalysis, renal
functions parameters (urea, creatinine and electrolytes) and renal
ultrasound. The diagnosis was made by micturating cystourethrogram (MCU)
and confirmed on cystourethroscopy.
The surgical treatment was cystourethroscopy and PUV
ablation; diversion was reserved for patients where ablation was not
advisable/feasible. Postoperatively, all patients received prophylactic
oral antibiotics and oxybutynin (0.2 mg/kg/day), which was discontinued
after toilet training, and when timed voiding was possible.
The renal function (nadir creatinine) was analyzed in
relation to oligohydramnios, gestational status at delivery, age at
intervention, mode of therapy (diversion/PUV ablation), presence of
vesicoureteral reflux (VUR) and preoperative infection. Initial
creatinine of more than 0.6 mg/dL was considered as raised, and patients
with nadir creatinine of more than 1.2 mg/dL at 6 months were considered
to be at risk of progression to chronic renal insufficiency.
Results
We managed 218 patients with PUV during the study
period. Ninety-two (42.5%) had antenatal hydronephrosis; 81 of these
were analyzed for this study (minimum of 6 months follow-up). Out of 81
children, 11 were excluded (2 died before treatment, one moved overseas
after treatment, and 8 patients had insufficient data).
The mean (SD) gestational age at antenatal diagnosis
was 34 (4.48) weeks, and mean (SD) age at intervention was 124 (147)
days. The mean (SD) follow-up period was 39.2 (27.6) months, and mean
(SD) age at last follow-up was 43.4 (28) months. Twenty-five patients
(33%) had VUR.
Sixty patients (85%) were managed by primary valve
ablation and 15% underwent diversion, of which eight have been
undiverted during follow-up. Two patients are still on diversion and two
of the diverted patients died of renal failure.
The mean (SD) initial and nadir creatinine was 0.87
(0.98) mg/dL and 0.41 (0.35) mg/dL, respectively. Initial creatinine was
raised in 24 (30%) patients. After treatment, 12 (15%) patients had
nadir creatinine >1.2 mg/dL with a trend towards higher creatinine on
follow-up (Fig. 1).
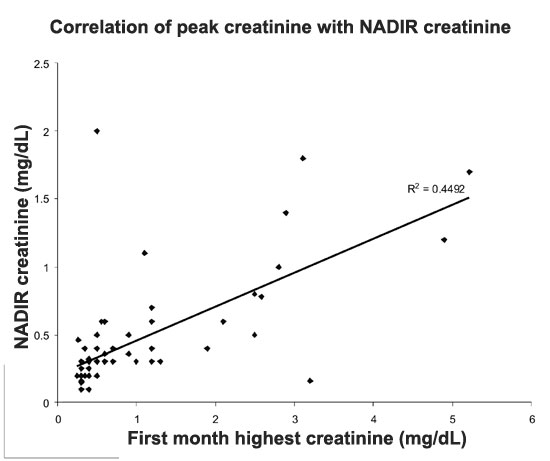 |
|
Fig. 1 The correlation between peak
creatinine and nadir creatinine over time.
|
Fifty-two patients could be assessed for voiding
function. Three patients underwent ablation of residual valve and four
of the toilet-trained patients had bladder dysfunction at last
follow-up. On nuclear renal scans, eight patients had poorly functioning
unilateral renal units (<10% function), and three underwent unilateral
nephroureterectomy for recurrent urinary tract infections (UTI).
Discussion
In our series of children antenatally diagnosed with
posteriod urethral valves, thirty percent presented with raised peak
creatinine (>0.6 mg/dL). Vesicoureteral reflux (VUR) was present in
one-third of patients. Majority of our patients were managed by
endoscopic valve ablation and 15% needed diversion due to persisting
sepsis or progressive renal failure with electrolyte abnormalities.
More than two-thirds of PUV are detected antenatally
[7], but this proportion is less in developing countries [8]. The
classical ultrasound features of PUV (hydronephrosis,
distended/thickened bladder with dilated posterior urethra and
oligohydramnios) are present in about one-third of scans [9]. As
antenatal ultrasonography is not specific for PUV [10], careful
postnatal evaluation is warranted.
The treatment is mainly endoscopic valve ablation,
and diversion is used for those with persisting sepsis or failed
endoscopic therapy. In a series of 65 cases of antenatally diagnosed PUV
[11], 97% were managed by valve ablation alone. Conservative management
is advocated for VUR in PUV as majority resolve with time. Heikkila,
et al. [12] reported that almost half of 197 patients with PUV had
resolution of VUR within 2 years after treatment.
The renal outcome of PUV is largely based on nadir
creatinine; a recent study [13] showed that nadir level after treatment
is reached by six months. The functional outcome is better for
prenatally diagnosed PUV [14], and bladder function improves with longer
follow-up.
PUV can present with antenatal hydronephrosis or
postnatally with bladder outflow obstruction. Endoscopic valve ablation
is the main modality of treatment and diversion is reserved if the
former fails or is contraindicated. The prognosis of patients with mild
disease and normal renal function is good, and in those with
intermediate severity disease, postnatal therapy improves the outcome.
Acknowledgements: Dr Sreekumaran MI, who
was part of the team in clinical management. Dr Biju George, for help in
statistical analysis.
Contributors: TPJ: clinical management of
patients, supervised the data collection and contributed to critical
review of the article; VKG: helped in manuscript editing and clinical
management; PRB: helped in manuscript editing and clinical management;
KVSK: Data collection, data analysis and manuscript drafting.
Funding: None; Competing interest: None
stated.
|
What This Study Adds?
• Prenatally diagnosed PUV has good functional outcome.
|
References
1. Belloli G, Battaglino F, Mercurella A, Musi L,
D’Agostino D. Evolution of upper urinary tract and renal function in
patients with posterior urethral valves. Pediatr Surg Int.
1996;11:339-43.
2. Farhat W, McLorie, Capolichio G, Khoury A, Bagli
D, Merguerian PA. Outcomes of primary valve ablation versus urinary
tract diversion in patients with posterior urethral valves. Urology.
2000;56:653-7.
3. Smith GH, Canning DA, Schulman SL, Snyder HM,
Ducket JW. Long term outcome of posterior urethral valves treated with
primary valve ablation and observation. J Urol. 1996;155:1730-4.
4. Heikkila J, Holmberg C, Kyllonen L, Rintala R,
Taskinen S. Long-term risk of end stage renal disease in patients with
posterior urethral valves. J Urol. 2011;186:2392-6.
5 Malin G, Tonks AM, Morris RK, Gardosi J, Kilby MD.
Congenital lower urinary tract obstruction: A population-based
epidemiological study. BJOG. 2012;119:1455-64.
6. Ruano R, Sananes N, Sangi-Haghpeykar H, Hernandez-Ruano
S, Moog R, Becmeur F. Fetal intervention for severe lower urinary tract
obstruction: A multicenter case-control study comparing fetal cystoscopy
with vesicoamniotic shunting. Ultrasound Obstet Gynecol. 2015;45:452-8.
7. Samnakay N, Orford J, Barker A, Charles A, Newnham
J, Moss T. Timing of morphologic and apoptotic changes in sheep fetal
kidney in response to bladder outflow obstruction. J Pediatr Urol.
2006;2:216-24.
8. Thakkar D, Deshpande AV, Kennedy SE. Epidemiology
and demography of recently diagnosed cases of posterior urethral valves.
Pediatr Res. 2014;76:560-3.
9. Holmes N, Harrison MR, Baskin LS. Fetal surgery
for posterior urethral valves. Long term postnatal outcomes. Pediatrics.
2001;108:E7.
10. Bhadoo D, Bajpai M, Abid A, Sukanya G, Agarwala
S, Srinivas M, et al. Study of prognostic significance of
antenatal ultrasonography and renin angiotensin system activation in
predicting disease severity in posterior urethral valves. J Indian Assoc
Pediatr Surg. 2015;20:63-7.
11. Sarhan O, Zaccaria I, Macher MA, Muller F,
Vuillard E, Delezoide AL, et al. Long-term outcome of prenatally
detected posterior urethral valves: Single center study of 65 cases
managed by primary valve ablation. J Urol. 2008;179:18-9.
12. Heikkilä J, Rintala R, Taskinen S. Vesicoureteral
reflux in conjunction with posterior urethral valves. J Urol.
2009;182:1555-60.
13. Deshpande AV, Alsaywid BS, Smith GH. Setting the
speed limit: a pilot study of the rate of serum creatinine decrease
after endoscopic valve ablation in neonates. J Urol. 2011;185:2497-500.
14. Kousidis G, Thomas DF, Morgan H, Haider N,
Subramaniam R, Feather S. The long-term outcome of prenatally detected
posterior urethral valves: a 10 to 23-year follow-up study. BJU Int.
2008;102:1020-4.
|
|
|
 |
|

