|
|
|
Indian Pediatr 2010;47: 309-315 |
 |
Single Dose Azithromycin Versus
Ciprofloxacin for Cholera in children: A Randomized
Controlled Trial
|
|
Jaya Shankar Kaushik, Piyush Gupta, MMA Faridi and Shukla Das*
From the Department of Pediatrics and *Department of
Microbiology, University College of Medical Sciences and
Guru Teg Bahadur Hospital, Dilshad Garden, Delhi, India.
Correspondence to: Dr Jaya Shankar Kaushik, 82-B,
Saraswati Kunj, Plot No 25, IP Extension,
Delhi 110 092, India. [email protected]
Received: August 27, 2008;
Initial review: October 13, 2008;
Accepted: March 27, 2009.
Published online:
2009.
May 20. PII:S097475590800521-1
|
|
Abstract
Objective: To compare the clinical and
bacteriological success of single dose treatment with azithromycin and
ciprofloxacin in children with cholera.
Design: Randomized, open labelled, clinical
controlled trial.
Setting: Tertiary care hospital.
Participants: 180 children between 2-12 years,
having watery diarrhea for £24 hr
and severe dehydration, who tested positive for Vibrio cholerae
by hanging drop examination or culture of stool.
Intervention: Azithromycin 20 mg/kg single dose (n=91)
or Ciprofloxacin 20 mg/kg single dose (n=89). Dehydration was
managed according to WHO guidelines.
Main outcome measures: Clinical success
(resolution of diarrhea within 24 hr) and bacteriological success
(cessation of excretion of Vibrio cholerae by day 3). Secondary
outcome variables included duration of diarrhea, duration of excretion
of Vibrio cholerae in stool, fluid requirement, and proportion of
children with clinical or bacteriological relapse.
Results: The rate of clinical success was 94.5%
(86/91) in children treated with Azithromycin and 70.7% (63/89) in those
treated with Ciprofloxacin [RR (95% CI)=1.34 (1.16-1.54); P<0.001].
Bacteriological success was documented in 100% (91/91) children in
Azithromycin group compared to 95.5% (85/89) in Ciprofloxacin group [RR
(95% CI)=1.05 (1.00-1.10); P=0.06]. Patients treated with
Azithromycin had a shorter duration of diarrhea [mean(SD) 54.6 (18.6)
vs 71.5 (29.6) h; mean difference (95% CI) 16.9 (9.6–24.2); P<0.001]
and lesser duration of excretion of Vibrio cholerae [mean(SD)
34.6 (16.3) vs 52.1 (29.2) h; mean difference (95% CI) 17.5 (0.2–24.7),
P<0.001] in children treated with Azithromycin vs Cipro-floxacin.
The amount of intravenous fluid requirement was significantly less among
subjects who received Azithromycin as compared to those who received
Cipro-floxacin [mean(SD) 4704.7(2188.4) vs 3491.1(1520.5) mL; Mean
difference (95% CI) 1213(645.3–1781.9); P<0.001]. Proportion of
children with bacteriological relapse was comparable in two groups [6.7%
(6/89) vs 2.2% (2/91); RR (95% CI) 0.95 (0.89–1.01); P=0.16].
None of the children in either group had a clinical relapse.
Conclusion: Single dose azithromycin is superior
to ciprofloxacin for treating cholera in children.
Key words: Azithromycin, Antibiotic, Cholera, Cipro-floxacin,
Management.
|
|
WHO recommends a 3-5 day course of
furazolidone, trimethoprimsulpha-methoxazole or erythromycin for treatment
of cholera in children; tetracycline may be used for those more than 8
years of age(1-3). However, strains of V. cholerae resistant to
these drugs have been identified in Bangladesh and elsewhere(4).
Ciprofloxacin was found to be effective in treatment of cholera with a
good in vitro activity, long half life, high stool concentration
after ingestion and safety for use in children(5). Single dose
ciprofloxacin has been widely studied in adults(6,7) but studies in
children(8) are limited. Its mechanism of action is different from
penicillin, erythromycin and tetracycline, hence it can be used for
organisms resistant to the traditionally recommended antibiotics. In
recent years, strains of V. cholerae resistant to fluoroquinolones
have also been identified from various parts of India(9-11).
Identification of clinically efficacious alternative antibiotics is
therefore necessary for use in children with cholera.
Azithromycin, a synthetic macrolide antibiotic is an
emerging antibiotic with action against V. cholerae(12). Single
dose treatment with azithromycin has a potential advantage of ease of
administration, good compliance, and reduced cost of treatment. Studies on
treatment of cholera in children with single dose azithromycin are limited
to comparisons with erythromycin(13,14). We compared the efficacy of
single dose of azithromycin to ciprofloxacin for treatment of cholera in
children and hypothesized that azithromycin is at least as effective as
ciprofloxacin in treatment of cholera.
Methods
The study was designed as a randomized, open labelled,
clinical controlled trial; and was conducted in a tertiary care hospital
of India, from March 2006 to February 2007. Clearance was obtained from
the institutional ethical committee. The study protocol was fully
explained to the parents/guardian, and informed written consent was
obtained.
Sample size: The sample size was calculated for
an equivalence study. Clinical success for treatment with single dose
ciprofloxacin was estimated at 94% in a previous study(8). To reach a
predictive power of 80%, with an alpha error of 5% and a beta error of
20%, 87 patients were required in each treatment group to show that the
difference in the rates of clinical success between the treatment groups
did not exceed 10%(15).
Enrolment: Children between 2-12 years, having
watery diarrhea for 24 hr or less, with features of severe dehydration as
per WHO criteria(3), were eligible to be included. Of these, only those
who demonstrated Vibrio cholerae in stool either by a
hanging drop preparation or culture, were finally analyzed. Children with
severe undernutrition (weight for age less than 60% of 50th percentile of
CDC 2000 standards), a coexisting systemic illness, blood in stool; and
those having received an antibiotic/antidiarrheal within preceding 24
hours, were excluded.
Data collection: Baseline data were collected:
this included name, age, address, telephone number, duration of illness,
frequency of diarrhea and vomiting prior to admission, and presence of
associated symptoms including abdominal pain, fever, and abdominal
distension. A history of previous antibiotic/antidiarrheal ingestion in
the last 24 hrs was elicited. Occupation, education and monthly income of
parents were recorded and a socioeconomic status was assigned based on
revised Kuppuswamy classification(16). Evaluation was done for general
hygiene, vitals, and signs of dehydration(2). The present weight was
recorded on a standardized weighing scale to the nearest 0.5 kg. Height
was measured to the nearest 0.1 cm. The same observer obtained all the
measurements.
Randomization and allocation: Eligible children
were allotted a study number. These numbers corresponded to the order of
patients entering the trial. Children were randomized to receive a single
dose of oral azithromycin (20 mg/kg) or ciprofloxacin (20 mg/kg). A simple
randomization was done using a computer generated random number table on a
master list.
Allocation of the treatment group was concealed by
having the names of both the study drug stored in identical sealed
envelope, which were opened after a patient had been enrolled in the study
and assigned a study number. Randomized children were immediately
rehydrated with intravenous Ringer’s lactate solution (30 mL/kg in first ½
hour followed by 70 mL/kg over next 2½ hours). A stool sample was obtained
for hanging drop examination and culture for Vibrio cholerae, as
soon as the child passed stools after admission. The patient was
reassessed for hydration after 3-4 hours and managed further as per the
WHO Guidelines(2).
The assigned Study drug was orally administered after
initial rehydration, under supervision. Eligible subjects received either
a single dose of azithromycin (20 mg/kg) or ciprofloxacin (20 mg/kg). Both
the drugs were available in 100 mg, 250 mg and 500 mg tablets and the dose
was rounded to nearest 50 mg. The dose was repeated if the child vomited
within 10 minutes of drug administration.
Each Study day was defined as 24 hour counted from the
administration of study drug. Children remained in the Study center for 72
hours (day 3) or until resolution of watery diarrhea, whichever was later.
The parents were asked to bring their child back for a follow-up visit on
day 7. If the patient failed to return on the follow-up visit, the parents
were contacted by telephone and asked to come on the next day.
Clinical monitoring was performed on multiple occasions
on the day of admission and subsequently at the end of day 1, 2, 3 and 7.
A record was kept of frequency of stool and vomiting for every 24 hrs. The
amount of intravenous fluid and ORS administered was also recorded at the
end of each Study day. A stool sample or rectal swab was obtained at the
end of day 1, 2, 3 and at follow-up visit (day 7). We also noted for any
possible adverse effects of the drug administered like hypersensitivity
reaction, phototoxicity, tendinopathy and joint pain or swelling.
Microbiological evaluation: The motility of
V. cholerae was seen by hanging drop prepara-tion(17). Stool sample
was transported in alkaline peptone water or Cary Blair media and
processed. The stool samples were cultured in bile salt agar, MacConkey
agar and thiosulphate citrate bile sucrose agar. Plates were incubated at
37ºC for 24 hours. The samples were inoculated in fresh alkaline peptone
water for enrichment and subseqent plating. Bacteriological analysis was
done by standard laboratory techinques(18) and V. cholerae isolates
were serotyped by slide agglutination test using specific antisera (Denca
Saken). Antimicrobial susceptibility testing of the strains was performed
by standard methods.
Outcome measures: The primary outcome variables
were (i) clinical success: defined as resolution of diarrhea
within 72 hours after the start of therapy; and (ii)
bacteriological success: defined as absence of Vibrio cholerae in
the stool sample from day 3 onwards. Resolution of diarrhea was considered
when the child has passed two consecutive formed stools or had not passed
stool for 12 hours.
Secondary outcome variables included (i) total
duration of diarrhea (recovery time) defined as time elapsed from the
entry into study till resolution of diarrhea in hours; (ii) total
requirement of ORS and/or intravenous therapy; (iii) duration of
excretion of V. cholerae in stool; (iv) proportion of
children with clinical relapse (defined when there was cessation of
diarrhea for 1 day or longer followed by return of diarrhea), or
bacteriological relapse (defined as a positive stool culture following a
negative culture report).
Statistical analysis: Data were analysed using
SPSS version 13.0. All quantitative variables (between the groups) were
compared by unpaired t-test; categorical variables were compared by
Chi-square test or Fisher’s exact test. P<0.05 was considered as
significant. Variables which were measured repeatedly were analysed with
repeated measure ANOVA at 1% level of significance to allow for multiple
comparisons.
Results
Four hundred seven children were included in the study
and were randomized to receive azithromycin (n=205) or
ciprofloxacin (n=202). Of these, 180 children who tested positive
for V. cholerae by hanging drop examination or culture of the stool
were finally included in the analysis, and designated as "Study subjects".
A total of 89 Study subjects received ciprofloxacin and 91 received
azithromycin (Fig. 1). Baseline characteristics of the study
subjects were comparable between the groups (Table I).
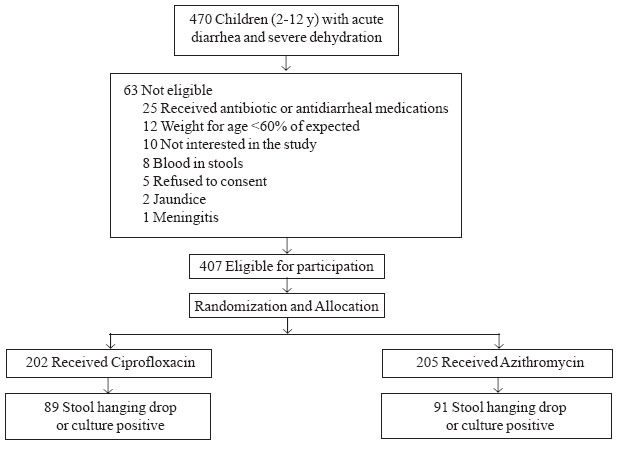 |
|
Fig. 1
Study Flow Chart.
|
TABLE I
Baseline Comparison of Patient Characteristics in the Two Groups
Patient
characteristics |
Ciprofloxacin
(n=89) |
Azithromycin
(n=91) |
P
value |
|
Mean (SD) |
| Age (months) |
64 (33.9) |
70 (37.7) |
0.24 |
| Weight (kg) |
17.4 (7.2) |
18.6 (7.7) |
0.25 |
| Height (cm) |
102.5 (17.8) |
105.3 (18.1) |
0.28 |
| Loose stools* |
15.2 (4.5) |
14.4 (4.7) |
0.24 |
| Duration of diarrhea (h) |
17.9 (7) |
18 (6.8) |
0.94 |
| Frequency of vomiting |
9.7 (6.1) |
9.7 (6.2) |
0.97 |
|
Proportion (%) |
| Male Sexs |
52 (58.4%) |
51 (56.1%) |
0.75 |
| Residence |
| Rural |
3 (3.3%) |
8 (8.8%) |
0.01 |
| Urban |
29 (32.5%) |
13 (14.2%) |
|
| Urban slum |
57 (64%) |
70 (76.9%) |
|
| Socioeconomic status (SES) |
| Lower SES |
19 (21.3%) |
20 (21.9%) |
0.17 |
| Middle SES |
51 (57.3%) |
41 (45.1%) |
|
| Upper SES |
0 (0%) |
1 (1.1%) |
|
| Source of drinking water |
| Treated |
61 (68.5%) |
68 (74.7%) |
0.36 |
| Untreated |
28 (31.5%) |
23 (23.3%) |
|
| Safe water
storage practices |
20 (22.5%) |
22 (24.1%) |
0.78 |
| Open field latrine |
25 (28.1%) |
25 (27.5%) |
0.93 |
| Flush latrine |
64 (71.9%) |
66 (72.5%) |
|
| Proper hand
washing |
53 (59.5%) |
54 (59.3%) |
0.97 |
| Children breastfed |
87 (97.7%) |
88 (96.7%) |
0.67 |
| Frequency of symptoms [n (%)] |
| Vomiting |
78 (87.6%) |
81 (89%) |
0.77 |
| Pain abdomen |
32 (35.9%) |
30 (32.9%) |
0.67 |
| Abdominal distension |
5 (5.6%) |
2 (2.2%) |
0.27 |
| Fever |
4 (4.4%) |
3 (3.2%) |
0.72 |
Means compared with Student’s t-test; proportions compared by Chi-square test/Fisher’s exact test; *Prior to admission.
|
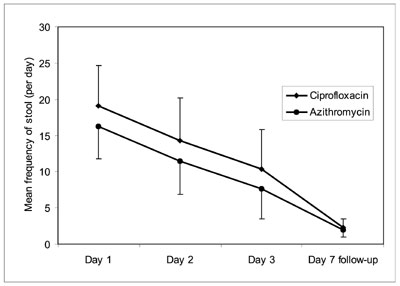
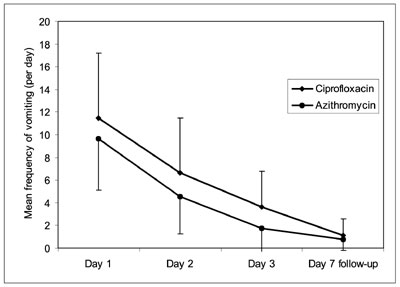
Outcome variables between the two groups are compared
in Table II. Symptomatic improvement was assessed by
comparing the frequency of diarrhea and vomiting. The frequency of stool
and vomiting was significantly lower in children who received azithromycin
as compared to the ciprofloxacin group during the first 72 hours. The rate
of decline in frequency of stool and vomiting was however comparable
between ciprofloxacin and azithromycin groups (Fig. 2a, 2b).
Table II
Comparison of Outcome Variables in Ciprofloxacin and Azithromycin Groups
| Outcome variable |
Ciprofloxacin (n=89) |
Azithromycin (n=91) |
Relative Risk(95% CI) |
P value |
| Number (%) |
| Clinical success |
63 (70.6%) |
86 (94.5%) |
1.33 (0.65–0.86) |
<0.001 |
| Bacteriological success |
85 (95.5%) |
91 (100%) |
1.04 (0.91–0.99) |
0.06 |
| Bacteriological relapse |
6 (6.7%) |
2 (2.2%) |
0.95 (0.89–1.01) |
0.16 |
| Clinical relapse |
Nil |
Nil |
– |
– |
| Mean (SD) |
Mean difference(95% CI) |
| Duration of diarrhea (h) |
71.5 (29.6) |
54.6 (18.6) |
16.9 (9.6–24.2) |
<0.001 |
| Duration of excretion of Vibrio cholerae
(h) |
52.1 (29.2) |
34.6 (16.3) |
17.5 (10.3-24.7) |
<0.001 |
| ORS requirement (mL) |
3473.8 (1341.7) |
3644.4 (1374.9) |
-170.6 (-577.6–236.3) |
0.41 |
| IV fluid requirement (mL) |
4704.7 (2188.4) |
3491.1 (1520.5) |
1213 (645.3–1782.0) |
<0.001 |
 |
 |
| (a) |
(b) |
|
Fig. 2 Comparison of mean frequency of (a)
diarrheal stools and (b) vomiting between Ciprofloxacin and
Azithromycin groups on day 1, day 2, day 3, and at follow-up visit
(day 7). |
The follow-up loss in first 72 hours of hospital stay
was only 3.3%. However, the follow-up loss beyond day 3 was 18.8%, which
was significant. An intention to treat analysis was used for subjects lost
to follow-up. Baseline patient characteristics were compared for subjects
lost to follow up (n=34) with those who completed the study (n=146)
.
Discussion
Our study concluded that single dose azithromycin is
superior to single dose ciprofloxacin for the treatment of cholera in
children. The rate of clinical success was significantly more in patients
treated with Azithromycin as compared to those treated with Ciprofloxacin,
although the rate of bacteriological success was comparable in the two
groups. Subjects who received Azithromycin had a significantly lesser
duration of diarrhea, shorter duration of excretion of V. cholerae,
and lower requirement of intravenous fluids. Rate of bacteriological
relapse was found to be comparable and none of the subjects in either
group had clinical relapse.
The results pertaining to superiority of single dose
azithromycin over ciprofloxacin are consistent with a previous study(7) in
adults. However, the rates of clinical and bacteriological success with
azithromycin are much higher in our study (95-100%) as compared to earlier
studies with azithromycin(7,13), which reported a success rate of between
70-75%. The discrepancy in the success rates could be attributed to
differing definitions of success adopted in these trials and differences
in baseline characteristics of the enrolled population. We adopted a 72
hours cut off for defining success instead of 48 hours used in the
previous studies(7,8,13). Another possible explanation is that the strains
of Vibrio cholerae in their study setting could have been earlier
exposed to azithromycin. This could lead to emergence of resistance to
azithromycin(19,20). As our study population was not exposed to the drug
for diarrheal illnesses, chances of V. cholerae exposure to the
drug were scanty, which could probably explain such high rates of clinical
and bacteriological success.
Quinolone antimicrobials, especially nalidixic acid,
are widely used in India for treatment of gastrointestinal infections.
Therefore, it could be expected that V. cholerae strains would have
received considerable exposure to these agents and exposure to nalidixic
acid could have been a selective force for quinolone resistance in India.
Hence, ciprofloxacin resistance might have emerged in direct response to
selective pressure exerted by nalidixic acid coupled with disproportionate
use of fluoroquinolones for all bacterial infections in our country. A
consistent increase in median inhibitory concentration (MIC) of V.
cholerae strains to ciprofloxacin has been reported(21,22). The
findings are troublesome as a further increase in MIC may render
ciprofloxacin ineffective in management of cholerae caused by such
multi-drug resistant strains of V. cholerae. Estimation of MIC in
our study could have answered many such queries. Considering the emergence
of fluoroquinolone resistance in our study setting(8), we could possibly
explain that although the sensitivity of ciprofloxacin reaches 99.4% in
our study, the strains probably required higher doses of ciprofloxacin and
for a longer duration to be clinically and bacteriologically effective.
The strength of our study was its robust design. The
sample was statistically sound, practical, suited the convenience and
provided credibility to our results. The only study on efficacy of
azithromycin for treatment of cholera in children has limitation of small
sample size(16), and this study did not analyse the outcome measures in
terms of clinical or bacteriological success. Our study setting was based
on a tertiary care hospital catering to all ailments of an urban
population. This ensures a true picture of diarrheal disease burden as
compared to referral centers catering to only diarrheal disease, as is the
case in most of the previous pediatric studies on cholera. Our study had
certain limitations; the intervention was not masked, there was moderate
follow-up loss and the volume of diarrhea and vomiting was not
ascertained. The population from urban slums is migratory, and it is
typical for them to change homes, which is primarily dictated by job
requirements. We acknowledge this as a reality for such trials conducted
in the developing world, accounting for our follow-up losses. However,
this did not affect the results as shown by comparable baseline
characteristics of study subjects and those lost to follow-up.
We conclude that single dose azithromycin is a useful
alternative for treating cholera in children. Considering the clinical
efficacy and lack of resistance to azithromycin, we advocate that it
should be considered as an option for first line treatment of childhood
cholera in areas where V. cholerae infection are caused by
susceptible strains.
Contributors: The study was conceived by PG. Data
were collected by JSK under the supervision of PG and MMAF; and analyzed
and interpreted by PG, JSK and SD. The article was drafted by JSK and PG.
The final version was approved by all authors.
Funding: None.
Competing interests: Azithromycin was provided by
FDC India limited.
|
What is Already Known?
• Azithromycin is efficacious for treatment of
cholera in adults.
What This Study Adds?
• Azithromycin is superior to ciprofloxacin for treatment of
cholera in children.
|
References
1. Gupta P, Ghai OP. Acute Diarrheal Diseases. In:
Gupta P, Ghai OP, editors. Textbook of Preventive and Social Medicine, 2nd
ed. New Delhi: CBS Publishers and Distributors; 2007. p. 298-305.
2. WHO Guidance on Formulation of National Policy on
the Control of Cholera. From http://www.who.int/topics/cholera/publications/WHO_CDD_
SER_92_16/en. Accessed on January 12, 2007.
3. World Health Organization. Guidelines for Cholera
Control. Geneva: WHO; 1993.
4. Emergence of a unique, multi-drug resistant strain
of Vibrio cholerae O1 in Bangladesh. From: http://www.icddrb.org/images/hsb32_Eng-emergence.pdf.
Accessed on August 20, 2006.
5. Grady R. Safety profile of quinolone antibiotics in
the pediatric population. Pediatr Infect Dis J 2003; 22: 1128-1132.
6. Khan WA, Michael LB, Seas C, Khan EH, Ronan A, Dhar
U, et al. Randomized controlled comparison of single dose
ciprofloxacin and doxycycline for cholera caused by V. cholerae O1
or O139. Lancet 1996; 348: 296-300.
7. Saha D, Karim MM, Khan WA, Ahmed S, Salam MA,
Bennish ML. Single-dose azithromycin for the treatment of cholera in
adults. N Engl J Med 2006; 354: 2452-2462.
8. Saha D, Khan WA, Karim MM, Chowdhury HR, Salam MA,
Bennish ML. Single-dose ciprofloxacin versus 12-dose Erythromycin for
childhood cholera: a randomized controlled trial. Lancet 2005; 366:
1085-1093.
9. Garg P, Chakraborty S, Basu I, Datta S, Rajender K,
Bhattacharya T, et al. Expanding multiple antibiotic resistance
among clinical strains of Vibrio cholerae isolated from 1992-97 in
Calcutta, India. Epidemiol Infect 2000; 124: 393-399.
10. Jesudason MV, Balaji V, Thomson CJ. Quinolone
susceptibility of Vibrio cholerae O1 and O139 isolates from Vellore.
Indian J Med Res 2002; 116: 96-98.
11. Das S, Goyal R, Ramachandran VJ, Gupta S.
Fluoroquinolone resistance in Vibrio cholerae O1: emergence of El
Tor Inaba. Ann Trop Paediatr 2005; 25: 211-212.
12. Jones K, Felmingham J, Ridgway G. In vitro
activity of Azithromycin (CP-62, 993), a novel macrolide, against enteric
pathogens. Drugs Exp Clin Res 1988; 14: 613-615.
13. Khan WA, Saha D, Rahman A, Salam MA, Bogaerts J.
Comparison of single-dose azithromycin and 12-dose 3-day erythromycin for
childhood cholera: A randomized double-blind trial. Lancet 2002; 360:
1722-1727.
14. Bhattacharya MK, Dutta D, Ramamurthy T, Sarkar D,
Singharoy A, Bhattacharya SK. Azithromycin in the treatment of cholera in
children. Acta Paediatr 2003; 92: 676-678.
15. Kim JS. Determining sample size for testing
equivalence. From: http://www.devicelink.com/mddi/archive/97/05/020. html.
Accessed on February 23, 2008.
16. Dasgupta R. Social and Behavioral Sciences in
Health. In: Gupta P, Ghai OP, editors. Textbook of Preventive and Social
Medicine, 2nd ed. New Delhi: CBS Publishers and Distributors; 2007. p.
622-644.
17. Pariija SC. Identification of Vibrio cholerae. In:
Pariija SC (ed). Textbook of Practical Microbiology, 1st ed. New Delhi:
Ahuja Publishing House; 2007. p. 181-183.
18. Chattopadhyay DJ, Sarkar BL, Ansari MQ,
Chakrabarthi BK, Roy MK, Ghosh AN, et al. New phage typing scheme
for Vibrio cholerae O1 biotype ELTOR strains. J Clin Microbiol
1993; 31: 1579-1585.
19. Khan WA, Seas C, Dhar U, Salam MA, Bennish ML.
Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin.
A double blind, randomized controlled trial. Ann Intern Med 1997; 126:
697-703.
20. Faruque ASG, Alam K, Malek MA, Khan MGY, Ahmed S,
Saha D. Emergence of multi-drug resistant strain of Vibrio cholerae
O1 in Bangladesh and reversal
of their susceptibility to tetracycline after two years. J Health Popul
Nutr 2007; 25: 241-245.
21. Garg P, Sinha S, Chakraborty R, Bhattacharya SK,
Nair GB, Ramamurthy T, et al. Emergence of fluoroquinolone-resistant
strains of Vibrio cholerae O1 biotype ELTOR among hospitalized
patients with cholera in Calcutta, India. Antimicrob Agents Chemother
2001; 45: 1605-1610.
22. Krishna BV, Patil AB, Chandrasekhar MR. Fluoroquinolone-resistant
Vibrio cholerae isolated during a cholera outbreak in India. Trans
R Soc Trop Med Hyg 2006; 100: 224-226.
|
|
|
 |
|

