|
|
|
Indian Pediatr 2021;58:572-575 |
 |
Impact of Comorbidities
on Outcome in Children With COVID-19 at a Tertiary Care
Pediatric Hospital
|
|
Dipti Kapoor, Virendra Kumar, Harish Pemde, Preeti Singh
From Department of Pediatrics, Lady Hardinge Medical College, New
Delhi.
Correspondence to: Dr Dipti Kapoor, Associate Professor,
Department of Pediatrics, Lady Hardinge Medical College,
New Delhi 110 001.
[email protected]
Received: February 09, 2021;
Initial review: March 09, 2021;
Accepted: May 14, 2021.
|
Objective: To study the various comorbidities and
their impact on outcome of severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2) infected children. Methodology: Review of medical
records of 120 children (58.4% males), aged 1 month to 18 years,
admitted between 1 March and 31 December, 2020 with at least one
positive RT-PCR test for SARS-CoV-2. Clinical and demographic variables
were compared between children with and without co-morbidities.
Results: 62 (51.7%) children had comorbidities. The most common
comorbidity was tuberculosis (32.3%) followed by other infections
(27.4%) and hematological (19.4%) conditions. Fever (89.2%) was the most
common clinical feature followed by respiratory (52.5%) and
gastrointestinal (32.5%) manifestations. There was no significant
difference in the severity of COVID illness, length of hospital stay and
adverse outcomes (ventilation and mortality) among children with and
without comorbidities. Conclusion: The presence of a comorbid
illness in pediatric inpatients with COVID-19 did not impact the illness
severity, length of hospitalization, ventilation requirement and
mortality.
Keywords: Mortality, Outcome, Tuberculosis, Ventilation.
|
|
T
he Severe Acute Respiratory Syndrome
Coronavirus-2 (SARS-CoV-2) pandemic has
evolved rapidly leading to a multitude of presentations and
variable severity, with substantial information regarding
clinical manifestations and outcomes of coronavirus disease
(COVID-19) in adults. It has been observed that presence of
comorbidities is associated with severe illness and worse
outcomes in adults infected with SARS-CoV-2 [1,2], but our
knowledge about clinical characteristics as well as outcomes of
COVID-19 infected children with comorbidities is limited.
Moreover, there is limited literature on the spectrum of
pediatric comorbidities and their outcome in association with
SARS-CoV-2 infection from our country, which tends to be
entirely different from those observed in the children from
developed countries [3]. This study was planned to examine the
effect of comorbidities with regard to disease presentation,
evolution and outcomes in children infected with SARS-CoV-2.
METHODS
This case record review was undertaken at a
tertiary care pediatric teaching hospital in northern India.
During the SARS-CoV-2 outbreak, any child brought with history
of recent onset fever, cough and/or fast breathing or other
suggestive symptoms like recent onset fever with diarrhea or
contact with COVID-19 positive patient was tested with RT-PCR
test for SARS-CoV-2. These children were also screened for
presence of any comorbidity. Comorbidity was defined as any
distinct additional acute or chronic condition that has existed
or may occur during the clinical course of a patient who has the
index disease under study, and might alter the course of disease
or the outcome [4].
Based on the results of confirmatory RT-PCR
and clinical assessment, cases were classified as asymptomatic,
mild, moderate and severe as per standard guidelines [5]. The
criteria for admission for suspected COVID-19 illness included
any of the following: respiratory distress, SpO2 on room air
<94, shock/poor peripheral perfusion, poor oral intake or
lethargy, specifically in infants and young children and/ or
presence of seizures or encephalopathy [6]. Based on the results
of confirmatory RT-PCR and clinical assessment, hospital
treatment or home isolation measures were instituted with
contact tracing measures as applicable (in accordance with the
local prevailing guidelines). The patients were managed as per
the standard WHO protocol [5].
All children 1 month to 18 years of age, with
at least one positive RT-PCR test for SARS-CoV-2 and requiring
admission between 1 March and 31 December, 2020 were included in
the study. A special COVID ward was created during the ongoing
pandemic for care of these children. Epidemiological,
demographic, clinical, treatment, and outcome data of children
with and without comorbidities was extracted from the case
records and compared. The study was reviewed and approved by the
institutional ethical committee with a waiver of consent for
data collection.
Statistical analysis: Comparison of means
between the two groups i.e., children with and without
comorbidities was performed using the two-sample Student t-test.
Categorical data were compared using Chi-square test. All tests
were 2-tailed with the threshold level of significance at P<0.05.
Statistical analysis was performed using STATA 14.2.
RESULTS
A total of 3180 suspected children were
tested for SARS-CoV-2; 295 (9.27%) children tested positive.
Amongst the latter, 120 SARS-CoV-2 positive children (70 boys)
required admission in the COVID ward. Fever (89.2%) was the most
common clinical feature at the time of presentation, and 64
(53.3%) had severe acute malnutrition or thinness. Comorbidities
were seen in 62 (51.7%) children. The most common comorbidities
were infections like tuberculosis (32.3%) followed by other
infections (27.4%) (Table I).
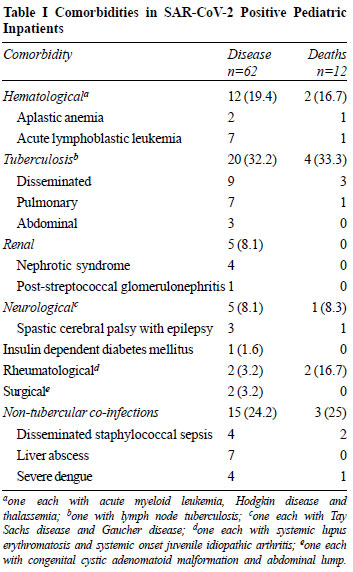 |
| |
The mortality rate in admitted patients was
24.2% (n=29). There was no difference in the clinical
characteristics of admitted children with and without
comorbidities with respect to baseline characteristics. There
was no statistical difference in the severity of COVID illness,
mean duration of hospital stay and adverse outcomes like
ventilation and mortality among the two groups (Table II).
However, severe anemia and thrombocytopenia were present in
significantly higher number of children with comorbidities (Table
II).
DISCUSSION
The clinical presentation in our cohort is
similar to that observed by previous studies [7]. There was no
significant difference in the clinical symptomatology, severity
of COVID illness, mean duration of hospital stay and adverse
outcomes among the children with and without comorbidities.
Tsankov, et al. [5] in their meta-analysis
observed that the most common comorbidity in children infected
with COVID-19 infection was obesity; whereas, we observed that
more than 50% of our patients were underweight for age. The
other comorbidities observed in their study were chronic
respiratory conditions, cardiovascular disorders and
neuromuscular diseases [5], whereas in our study where other
infections were the most common comorbidities. The mortality
rate observed in our study is higher than that reported globally
[7], presumably due to referral bias due to our center being one
of the largest tertiary care pediatric hospital in the public
sector in this region.
There is a dearth of published literature on
outcome of SARS-CoV-2 infected children with various
comorbidities [8-11]. Three children with disseminated
tuberculosis developed acute respiratory distress syndrome
(ARDS) and multi-organ failure syndrome (MODS), and one died due
to raised intracranial tension with neurogenic shock. The
children with hematological disorders died secondary to febrile
neutropenia with associated septicemia and catecholamine
refractory shock. One child with spastic cerebral palsy was
admitted with severe pneumonia and status epilepticus and went
on to develop ARDS. The patient with systemic lupus
erythematosus developed severe pneumonia with ARDS and acute
kidney injury, whereas the patient with systemic onset juvenile
idiopathic arthritis died due to macrophage activation syndrome
and MODS. Two children died due to disseminated staphylococcal
infection with catechola-mine refractory shock, and one due to
severe dengue with disseminated intravascular coagulation. The
mortality was not significantly different across groups,
possibly because most of our patients with comorbidities were
under regular follow-up in our hospital and were well versed
with the system, they might have presented early or might had
been diagnosed early with symptoms of COVID-19 infection.
Alternatively, some of these children were on immunomodulatory
and immunosuppressant drugs, which could also have modified the
course of infection by interfering with the cytokine storm
responsi-ble for organ damage in COVID-19 [12]. Most of the
SARS-CoV-2 infected children without comorbidities presented in
advanced and decompensated clinical condition, pre-sumably
secondary to suboptimal management caused by delay in diagnosis,
initiation of appropriate treatment, referral or transport
during this unprecedented time of ongoing pandemic. However,
this observation needs to be further evaluated in prospective
studies with larger sample size.
Tsankov, et al. [5] in their meta-analysis
also concluded that they could not determine whether
comorbidities increase risk of severe COVID-19 in children.
However, our observations are in contrast to those observed by
Rao, et al. [13], who observed that presence of comorbidity
increases the severity of COVID-19 disease.
Our study had limitations of having a
retrospective design, small sample size and lack of follow-up.
In spite of these shortcomings, this study provides preliminary
data on the spectrum and outcome of comorbidities in children
infected with SARS-CoV-2.
To conclude, the most common comorbidities
observed in COVID infected children were infections like
tuberculosis and other co-infections. There was no significant
increase in the severity of COVID illness, duration of hospital
stay or adverse outcome in these children. However, this
observation does not understate the vulnerability of these
children to develop severe illness and they should take all
necessary precautions to avoid getting infected with SARS CoV-2.
Further studies examining the effects of specific well-defined
comorbidities are warranted to examine the effects that
pediatric underlying conditions play in COVID-19 severity.
Ethical clearance: Institutional
Ethical Committee, LH Medical College; No. LHMC/IEC/2020/97,
dated November 6, 2020.
Contributors: DK: collected data and
wrote the initial manuscript; VK, PK: critically analyzed the
manuscript; PS: helped in data collection and revision of
manuscript. All the authors read and approved the final
manuscript.
Funding: None; Competing
interests: None stated.
|
WHAT THIS STUDY ADDS?
• Presence of a comorbid illness was
not associated with increase in the severity of COVID
illness, length of hospital stay or adverse outcome in
children.
|
REFERENCES
1. Guan W, Liang W, Zhao Y, et al.
Comorbidity and its impact on 1590 patients with COVID-19 in
China: A nationwide analysis. European Respiratory J. 2020; 55:
2000547
2. Zhou F, Yu T, Du R, et al. Clinical course
and risk factors for mortality of adult in-patients with
COVID-19 in Wuhan, China: A retrospective cohort study. The
Lancet. 2020; 395:1054-62,
3. Tsankov BK, Allaire JM, Irvine MA, et al.
Severe COVID-19 Infection and pediatric comorbidities: A
systematic review and meta-analysis. Intern J Infect Dis. 2021;
103: 246-56.
4. Valdres J M, Starfield b, Sibbald B, et
al. Defining comor-bidity: implications for understanding health
and health services. Ann Fam Med. 2009; 7: 357-63.
5. Clinical management of severe acute
respiratory infection when COVID-19 is suspected. Accessed
October 31, 2020. Available at:
https://www.who.int/publications-detail/clinical-management-of-severe-acuterespiratory-infection-when-novel-coronavirus-(ncov)-infection-issuspected
6. Dong Y, Mo X, Hu Y, et al. Epidemiological
characteristics of 2143 pediatric patients with 2019 coronavirus
disease in China. Pediatrics. 2020; e20200702.
7. Meena J, Yadav J, Saini L, et al. Clinical
features and outcome of SARS-CoV-2 infection in children: A
syste-matic review and meta-analysis. Indian Pediatr. 2020; 57:
820-26.
8. Shrinivasan R, Rane S, Pai M. India’s
syndemic of Tuberculosis and COVID-19. BMJ Global Health. 2020;
5: e003979.
9. Girmenia C, Gentile G, Micozzi A, et al.
COVID-19 in patients with hematologic disorders undergoing
therapy: Perspective of a large referral hematology center in
Rome. Acta Haematol. 2020; 143: 574-82.
10. Adeiza SS, Shuaibu AB, Shuaibu GM. Random
effects meta-analysis of COVID-19/S. Aureus partnership in
co-infection GMS. Hygiene and Infection Control. 2020;15:
2196-5226.
11. Saddique A, Rana MS, Alam MM, et al.
Emergence of co-infection of COVID-19 and dengue: A serious
public health threat. J Infect. 2020;81:16-18.
12. Collange O, Tacquard C, Delabranche X, et
al. Coronavirus disease 2019: associated multiple organ
damage. Open Forum Infect Dis. 2020;7:249.
13. Rao S, Gavali V, Prabhu S, et al. Outcome of children
admitted with SARS-CoV-2 infection: Experiences from a pediatric
public hospital. Indian Pediatr. 2021;58:358-62.
|
|
|
 |
|

