|
clinicopathological conference |
|
|
Indian Pediatr 2017;54: 765-770 |
 |
Hepatic
Sinusoidal-obstruction Syndrome and Busulfan-induced Lung Injury
in a Post-autologous Stem Cell Transplant Recipient
|
|
Richa Jain, *Kirti Gupta,
#Anmol Bhatia, Arun Bansal
and Deepak Bansal
From Departments of Pediatrics (Hematology-Oncology
Division), *Histopathology and Radiodiagnosis; PGIMER, Chandigarh,
India.
$Correspondence to: Dr Kirti Gupta,
Professor, Department of Histopathology, Postgraduate Institute of
Medical Education and Research (PGIMER), Chandigarh. India.
[email protected]
Received: June 23, 2016;
Initial Review: October 13, 2016;
Accepted: April 25, 2017.
|
Veno-occlusive disease of the liver
is mostly encountered as a complication of hematopoietic stem cell
transplantation with myeloablative regimens with an incidence estimated
to be 13.7%. It is clinically characterized by tender hepatomegaly,
jaundice, weight gain and ascites. Strong clinical suspicion and an
early recognition of clinical signs are essential to establish the
diagnosis and institute effective regimen. Another complication of
cytotoxic drugs given for cancers, is development of busulfan-induced
lung injury. A strong index of suspicion is needed for its diagnosis,
especially in setting where opportunistic fungal and viral infections
manifest similarly. We illustrate the clinical and autopsy finings in a
2˝-year-old boy who received autologous stem-cell transplantation
following resection of stage IV neuroblastoma. He subsequently developed
both hepatic veno-occlusive disease and busulfan-induced lung injury.
The autopsy findings are remarkable for their rarity.
Keywords: Autopsy, Hepatic veno-occlusive
disease, Neuroblastoma.
|
|
Clinical Protocol
A 2.5-year-old boy with stage IV neuroblastoma
(primary tumor in the left adrenal, with bone marrow and multiple bone
metastases) was undergoing therapy at our institute. The patient was
treated with 8-cycles of induction chemotherapy, consisting of
vincristine, carboplatin, cisplatin, cyclophosphamide and etoposide, as
per the SIOP high-risk neuroblastoma protocol. Chemotherapy was
administered at 10-day intervals with G-CSF prophylaxis. Induction was
uneventful, with no episodes of febrile neutropenia. A re-evaluation
bone marrow examination performed post-induction demonstrated a complete
response. At this stage, tumor excision along with left nephrectomy was
performed with resection of approximately 95% of the tumor. On
histology, it was categorized as differentiating neuroblastoma.
Post-surgery, patient was taken up for high-dose chemotherapy with
autologous stem cell transplantation (auto-SCT). Myeloablation was
performed with busulfan and melphalan with dose adjustment for weight
(10 kg). Busulfan was administered orally with a cumulative dose of 480
mg/m 2 and melphalan (120
mg/m2) was administered on
day -1. Busulfan level monitoring was not performed due to
non-availability. Prophylaxis for sinusoidal obstruction syndrome (SOS)
was ensured with ursodiol. The dose of CD-34+ cells infused was 4 × 106/kg.
The child had a relatively uneventful post-transplant period with fever
and mild mucositis lasting for 2 days. Neutrophil engraftment occurred
on day 13; however, platelet engraftment had not occurred by day 35. He
was discharged on day 14, on ursodiol prophylaxis. Pneumocystis
jirovecii (PCJ) prophylaxis had not been initiated on discharge.
On day 35, he presented with abdominal distension,
icterus (serum total bilirubin 35mmol/L) and weight gain of 2.5% above
the baseline. He had tender hepatomegaly (3 cms below costal margin). He
remained afebrile and non-neutropenic. Abdominal sonography and Doppler
demonstrated moderate ascites and left pleural effusion. The hepatic and
portal venous flow was normal. Liver and kidney functions were
unremarkable. A diagnosis of mild sinusoidal obstruction syndrome (SOS)
was made as per Seattle criterion [1]. He was managed with supportive
care (sodium and fluid restriction, ursodiol and spironolactone) as an
in-patient for 2 days, after which he improved and could be discharged.
He was well, and was being assessed on an OPD basis at weekly intervals
till day +60, when he presented with cough, tachypnea and hypoxia. The
illness started with respiratory distress two days prior to
presentation. The cough was non-productive and non-paroxysmal. There was
absence of fever, rash, neurological involvement and/or bleeding.
Clinical examination
Triage examination at day +60 showed an open and
stable airway, tachypnea, (respiratory rate 60/minute) with increased
efforts, hypoxia (saturation on room air: 90%; increasing to 95% on 40%
FiO2). Tachycardia was present (heart rate 132/minute); however,
circulatory parameters were normal (BP 98/54 mm Hg, normal capillary
refill time and pulse pressure, warm extremities). Pallor was present,
with no evidence of skin or mucosal bleeding. Systemic examination was
consistent with pneumonitis as the patient had tachypnea, bilateral
equal air entry, and presence of coarse crepitations in bilateral lung
fields. Abdominal examination showed dark brown pigmentation over
abdomen with no tenderness, guarding or rigidity. Hepatomegaly was
present with liver palpable 3 cm below costal margin (span 8 cm). Spleen
was not palpable. There was no free fluid. Cardiac and neurological
examinations were essentially normal.
Course and management
The index case was managed as a case of pneumonitis
post auto-SCT. Respiratory support was initially provided with nasal
prongs-continuous positive airway pressure. Intravenous antibiotics were
started cefoperazone-sulbactam, amikacin and azithromycin. Due to
rapidly progressive respiratory distress, child was transferred to
Pediatric intensive care unit where mechanical ventilation was provided.
There was single episode of fever on the day of admission (38.7 oC)
with a subsequent afebrile period throughout the hospital stay.
Progressive respiratory distress worsened into acute respiratory
distress syndrome (ARDS). Ventilation strategy was modified accordingly.
On day 63, there was development of hypotensive shock, initially
responding to fluid boluses. On day 65, the shock necessitated inotropic
support (dopamine, adrenaline, noradrenaline and pre-terminally, and
vasopressin). There was development of left sided pneumothorax followed
by cardiac arrest on the same day. The patient could be revived and
pneumothorax was drained. Multi-organ dysfunction developed with acute
kidney injury (onset day 63), requiring peritoneal dialysis. Significant
transaminitis with elevated bilirubin levels was documented. Antibiotics
were changed to vancomycin and meropenam on day 63; azithromycin was
continued. Intravenous co-trimoxazole and gancyclovir were added in
therapeutic, renal modified doses. Platelet concentrates were transfused
to maintain a platelet count above 20 × 106/mm3.
There was development of refractory shock on day 67. The child suffered
another cardiac arrest on day 68, and could not be revived.
Investigations
The hematological and biochemical investigations are
outlined in WebTable I. Echocardiography on day 62 showed
normal cardiac function with no evidence of pulmonary hypertension.
Coagulopathy was present (day 68) with elevated PT: 52s (normal 12-14
s); aPTT: 69s (normal: 33-36 seconds); with an INR of 3.67.
Microbiological analysis: Multiple blood
cultures (thrice) and urine culture (once) were sterile. Endotracheal
aspirate (ETA) on day 63 and day 64 showed presence of yeast. Serum
Galactomannan was negative. Both ETA and gastric aspirates were negative
for PCJ and acid fast bacilli (AFB) on two occasions. Pleural fluid
examination was unremarkable. Qualitative cytomegalovirus (CMV) PCR was
positive in ETA but negative in blood.
Radiology findings (Radiologist): Initial
few radiographs revealed ill-defined reticulo-nodular opacities in
bilateral lung fields (left > right) with relative sparing of bilateral
upper lobes and periphery. Subsequently, serial chest radiographs
revealed progressive pulmonary lesions on day 63 and day 65. A chest
radiograph on day 65 revealed pneumothorax, subtle pneumo-mediastinum
and subcutaneous emphysema. Expansion of left side of lung on the
radiograph done on day 66 was noted. Radiograph done on the day of
demise revealed significant increase in subcutaneous emphysema involving
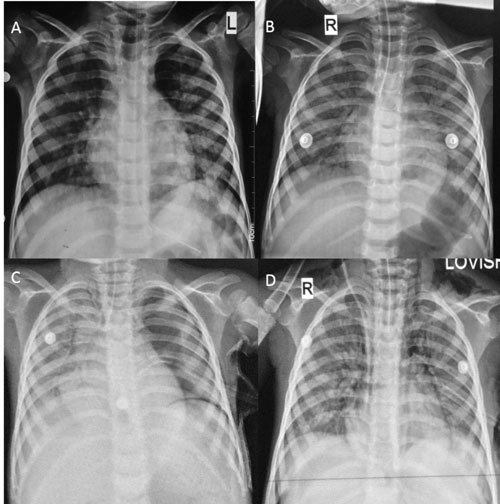
bilateral lower cervical region (Fig. 1a-d).
 |
|
Fig. 1 (A) Chest X-ray (CXR) showing
ill-defined reticulonodular opacities in both lungs, which
significantly increased in the CXR done after 48 hours (B); (C)
Follow-up CXR depicting pneumothorax on the left side, for which
a drainage tube was inserted; (D) Repeat CXR after 48 hours
demonstrates expansion of lung on left side with persistent
opacities in bilateral lungs. Pneumomediastinum and subcutaneous
emphysema can also be seen.
|
With these chest radiographs, possible etiologies
considered were infective, including fungal pneumonia and PCJ pneumonia,
CMV disease and miliary tuberculosis (TB). Non-infective etiologies
under consideration included pulmonary graft-versus host disease (GvHD)
and pulmonary veno-occlusive disease (VOD).
Discussion (Clinical Discussant)
This is a case of neuroblastoma, stage IV, day 68
post auto-SCT, presenting with fever, pneumonia, hypoxia, and
investigations showing polymorphic leucocytosis with deranged liver
function tests (LFT). In a post-transplant patient, complications can be
divided according to the duration subsequent to transplant. In the
initial 30 days, there is presence of neutropenia; between 30-100 days
is the early post-engraftment phase and beyond 100 days is late
post-engraftment phase.
The index case developed symptoms in the early post
engraftment phase. Common complications seen in early post-engraftment
phase can be divided into infective and non-infective. Infective
etiologies include CMV which can explain both pneumonia and hepatitis.
It is a common pathogen causing disease 3 weeks post SCT. India is an
endemic country for CMV. In the index case, ETA demonstrated polymerase
chain reaction (PCR) positivity for CMV along with radiological findings
which were supportive of the diagnosis. However, the child deteriorated
despite administration of gancyclovir from day 2 onwards, which is
unusual. Typically blood PCR is positive in such cases, though not
mandatory for diagnosis of CMV pneumonia. Fungal infections are the next
possibility, supported by the presence of candida in ETA on two
occasions. Presence of normal neutrophil count and a normal serum
Galactomannan are odd points. Galactomannan <0.5 has shown a good
negative predictive value for Aspergillus infection [2]. Other viral
infections that are important in post-SCT scenario include Respiratory
syncytial virus (RSV), Para-Influenza virus, Influenza, Metapneumovirus,
and Coronavirus. Multiorgan failure and lymphopenia is common in these
patients. Patients with RSV often require ventilation. Associated
co-infection with fungus, especially aspergillus can be seen. In the
absence of investigations directed towards the myriad respiratory
viruses, it is difficult to rule in or rule out these infections.
Tuberculosis should be considered in an immunocompromised patient in an
endemic country; however, the rapid onset of disease, absence of a
contact and negative evaluation make it unlikely. In our case other
bacterial infections typically seen in an immunocompromised child are
also unlikely in view of sterile cultures, complete absence of fever and
normal C-reactive protein (CRP).Though this clinical presentation can be
caused by infection with PCJ, it is an uncommon infection. Other
atypical infections like Nocardia and Cryptococcus are rarer still. The
non-infective etiologies causing respiratory symptoms in a
post-transplant setting can be pulmonary GvHD, Idiopathic pneumonia
syndrome (IPS), Bronchiolitis obliterans syndrome (BOS), Cryptogenic
organising pneumonia (COP) and SOS. IPS is a very common disease in this
situation, but is typically seen post allo-SCT and hepatitis is not an
associated feature. On-going hepatic SOS is unlikely as there was no
weight gain or tender hepatomegaly. GvHD and BOS are also typically
diseases seen in allo-SCT setting. Pulmonary SOS is very rare and normal
echo findings negate this possibility. The clinical presentation is
consistent with COP, though it is more common in females undergoing
allogenic transplant.
The final diagnosis is neuroblastoma stage IV, day +
68 post auto-SCT (Bu-Mel) with pneumonitis, ARDS and multi-organ
failure; likely etiology being fungal pneumonia or CMV pneumonia and
hepatitis secondary to ischemia with underlying SOS.
Pediatrician I: This patient presented in
2nd month post auto-SCT. Lymphocytes are still not functional at this
stage. Moreover, he had documented lymphopenia. There is a high
likelihood of infective causes with CMV and PCJ pneumonia. Other
important etiologies are Busulfan- induced lung injury, which typically
occurs in 2 nd month
post-transplant. He had received conditioning regimen of
melphalan-busulfan.
Pediatric hemato-oncologist 1: IPS occurs
post SCT day+60 to 80. This child had typical bilateral basilar
infiltrates and hypoxia. Moreover, IPS has a relationship with use of
busulfan and pre-existing SOS. Presence of CMV positivity in ETA is of
questionable significance as it is a common organism. Histopathological
evidence from lung biopsy is essential to prove CMV pneumonia. Liver
dysfunction in the form of transaminitis was likely due to shock and
ischemia.
Pediatric hemato-oncologist 2: Bacterial
and fungal infections cannot be excluded despite absence of fever,
several sterile cultures and continued normal values of CRP, though less
likely. However, both CMV and PCJ infections are possible with normal
CRP. Absence of adventitious lung sounds at initial presentation, along
with presence of hypoxia may be a pointer towards PCJ pneumonia.
Immunocompromised state, lymphopenia and the fact that the child was not
on PCJ prophylaxis are important here. Moreover, CMV is ubiquitous in
our pediatric population, and in pediatric oncology patients, we have
seen a near 100% seropositivity. Reactivation of CMV can occur at any
point of time in these patients. Important non-infective possibilities
are IPS and cryptogenic pneumonia. Pulmonary SOS is quite unlikely given
the normal echo findings.
Adult hematologist: Immune reconstitution
post-transplant takes typically 6 to 12 months. This child was
immunocompromised. Adenovirus infection can be considered. It can be
rarely seen in association with hepato-pulmonary syndrome.
Pathology Protocol
The excised tumor on histology was categorized as
differentiating neuroblastoma (Fig. 2a-c). Autopsy
revealed normal serous cavities. Liver weighed 290 g and revealed
irregular areas of sinusoidal congestion, confluent at places with
necrosis of adjoining parenchyma involving both right and left lobe (Fig.
2d). No thrombi were identified in right and left hepatic
vein or inferior vena cava. Microscopically, areas of centrizonal
congestion were identified (Fig. 2e). Furthermore,
the dominant pathology was seen in the central vein and terminal hepatic
venule (THV). There was varying degree of obliterative changes with
subendothelial fibrosis and laying down of reticulin fibres and
collection of extracellular matrix in subintimal zone. At places, the
THV was completely obliterated with wipe out of centrizonal hepatocytes
while the periportal hepatocytes were preserved (Web Fig. 1A-E).
In other regions, extravasated RBCs and areas of hemorrhage were noted
in centri-zonal regions. Besides acute obliterative changes, subacute
changes in form of deposition of collagen around the hepatic venule and
collection of hemosiderin laden macrophages were also noted. Loss of
hepatic parenchyma resulted in approximation of central veins structures
(Web Fig. 1A).
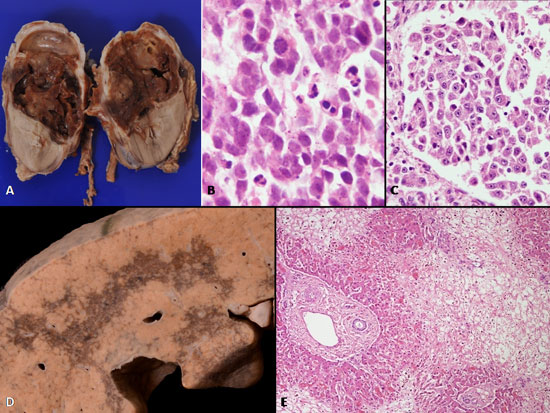 |
|
Fig. 2 (A) Gross of resected tumor at
the upper pole of the left kidney. It is largely necrotic; (B)
Round to oval cells with high N/C ratio with abundant mitoses
and apoptotic debris (H&E × 400); (C) About 40% of cells
demonstrated nodular areas of differentiating cells of large
size, with nucleolar prominence and moderate cytoplasm (H&E ×
200); (D) Gross of live showing haemorrhagic discoloration
involving both right and left lobe; (E) Areas of loss of hepatic
parenchyma with congestion in the centrizonal areas, only the
peri-portal areas are preserved
(H&E × 100).
|
Both lungs weighed 245g with dull pleura. Microscopy
revealed features of busulfan-induced lung injury with marked prominence
of Type II pneumocytes; many of them demonstrated nuclear atypia and
hyperchromasia. Marked thickening of interstitium with fibrosis was also
noted (Web Fig. 2A-B). Other regions showed patchy
acute bronchopneumonia and alveolar haemorrhages. Features of pulmonary
arteriopathy were also noted with prominence of intra-acinar arterioles.
There were no features of VOD in the pulmonary veins. An occasional
focus of septic emboli with Candida infiltration into parenchyma was
noted. No CMV inclusions were noted in lungs. PCR carried out on lung
tissue for adenovirus, RSV and metapneumovirus were negative.
Acute ulcers with Candida infiltration were noted in
stomach and small intestine. Candida had disseminated to heart causing
mural endocarditis, myocardial abscess and tiny (2-4 mm) vegetations on
left atrial wall (WebFig. 2 C-F). Both tricuspid and
mitral valves were normal. Dissemination with formation of fungal
abscesses were also detected in psoas muscle and omental fat. Subsequent
to septic emboli, infarcts were detected in right kidney (upper pole)
with thrombi within the branches of renal vessels and spleen. Right
kidney also revealed features of acute tubular necrosis in non-infracted
regions. No residual tumor was detected in lymph nodes, thymus and bone
marrow.
The autopsy diagnosis is concluded as follows:
In a known case-of neuroblastoma, undifferentiated
(adrenal) post-autologous stem-cell transplant:
• Features of busulfan-induced lung injury with
organizing bronchopneumonia and pulmonary arterial hypertension;
• Veno-occlusive disease in liver.
• Fungal (Candida) ulcers in GIT with extensive
dissemination to heart (mural endocarditis and myocardial abscess),
lungs, skeletal muscle and omental fat producing embolic infarcts in
right kidney and spleen.
• No residual disease in bone marrow.
Discussion
Hepatic sinusoidal obstruction syndrome (SOS) is an
obliterative venulitis of THV which occurs as a result of cytoreductive
therapy prior to hematopoietic stem cell transplantation (HSCT),
ingestion of pyrrolizidine alkaloids, or radiation therapy [3-6]. The
primary pathogenetic event is the endothelial injury of sinusoids and
small hepatic veins. Following which, there is deposition of
fibrin-related aggregates and oedema in the subendothelial zone [3].
Accumulation of these aggregates and entrapment of fluid and cellular
debris progressively occlude the hepatic venous flow and leads to
post-sinusoidal intrahepatic hypertension. This is accompanied by
necrosis of perivenular hepatocytes. Histologically, acute, sub-acute
and chronic forms of SOS have been described depending upon
collagenization and fibrosis of terminal hepatic venule. Incidence of
SOS varies from 0-70%, as it depends on the conditioning regimen used as
well as upon the patient’s risk factors [4-6]. SOS occurs more often
after allo- than after auto-HSCT (8 v/s 3%, respectively), suggesting a
role of immune reactions in this disorder [7]. Few independent studies
have documented increase in circulating levels of plasminogen activator
inhibitor-1 (PAI-1), a molecule released by the endothelial cells, in
patients developing SOS [8,9]. Increased PAI-1 levels might be of
clinical utility in challenging clinical situations in patients with
hyperbilirubinemia occurring after HSCT. It forms one of the therapeutic
targets for defibrotide, which reduces circulating PAI-1 levels along
with other actions. Other endothelial markers, like intercellular
adhesion molecule-1 (ICAM-1), E-selectin, von Willebrand factor (VWF),
and thrombomodulin may also be helpful in early identification of
patients at risk of SOS who may benefit from early introduction to
therapies [10]. Diagnosis of SOS is based on constellation of signs and
symptoms and serum bilirubin levels. Hepatic SOS is clinically
characterized by jaundice caused mainly by conjugated hyperbilirubinemia,
tender hepatomegaly, fluid accumulation manifested as rapid weight gain
and ascites [4]. Most commonly used diagnostic criteria for SOS includes
the Seattle criteria [11], the modified Seattle criteria [1], and the
Baltimore criteria [12]. Because of its high incidence and mortality,
prophylaxis for hepatic SOS is widely practiced, using different
regimens in different centres. When hepatic SOS is established, specific
therapy is usually given in addition to general supportive care,
especially in moderate or severe cases. Hepatic SOS is a formidable
challenge both for patients undergoing stem cell transplantation and for
their physicians.
The second pathology in this child which
significantly contributed to his downhill course was busulfan induced
lung injury. Intriguingly, in the present clinical setting, busulfan
induced lung injury remains an diagnosis of exclusion, particularly with
respect to considering usual and atypical infections. Its clinical
presentation includes a spectrum ranging from acute, rapidly progressive
respiratory distress to chronic, interstitial lung disease with
insidious onset [13,14]. The pathophysiology of drug-induced lung injury
is not fully understood but direct toxicity of the drug to parenchymal
cells, cell-mediated immune reactions and release of cytokines are
believed to contribute to the lung injury. The pathologic findings
consist of mainly diffuse interstitial pneumonitis, organizing
alveolitis and cellular atypia within type II pneumocytes. The injury
pattern with busulfan is diffuse alveolar damage (DAD) either in acute
exudative phase with alveolar and interstitial oedema and hyaline
membranes; or late reparative phase, which is characterized by
proliferation of type II pneumocytes and interstitial fibrosis [15].
Marked atypia of the type II pneumocytes is a morphological clue in
favour of busulfan induced lung injury in contrast to organizing
bacterial pneumonia. Moreover, PCR for CMV is helpful in excluding viral
pneumonia.
The prevalence of drug-induced pulmonary toxicity is
increasing, and more than 100 drugs are now known to cause lung injury.
Because this lung injury can be progressive and fatal, early recognition
is important. The diagnosis of pulmonary drug toxicity should be
considered in any patient with a history of drug therapy who presents
with new or progressive respiratory complaints.
The superadded fungal ulcer which developed
preterminally with extensive dissemination to heart causing mural
endocarditis and myocardial abscess eventually led to the demise of the
child.
Hepatic SOS contributes considerably to
transplantation-related morbidity and mortality. Recognition of this
disease in the post-transplantation setting remains a challenge in the
absence of specific diagnostic features as many other more common
conditions can mimic it. A high index of suspicion is needed to identify
patients with SOS. While hepatic SOS and busulfan induced lung injury
are commonly reported as isolated findings following autologous SCT, the
co-existence of these are extremely rare and have not been documented in
the literature thus far. The present case adds observational data to the
existing literature and highlights the importance of keeping high index
of suspicion for these two entities in patients following HSCT, and
early institution of effective therapy.
References
1. McDonald GB, Hinds MS, Fisher LD, Schoch HG,
Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver
and multiorgan failure after bone marrow transplantation: a cohort study
of 355 patients. Ann Internal Med. 1993;118:255-67
2. Jha AK, Bansal D, Chakrabarti A, Shivaprakash MR,
Trehan A, Marwaha RK. Serum galactomannan assay for the diagnosis of
invasive aspergillosis in children with haematological malignancies.
Mycoses. 2013;56:442-8.
3. Fan CQ, Crawford JM. Sinusoidal obstruction
syndrome (hepatic veno-occlusivedisease). J Clin Exp Hepatol.
2014;4:332-46.
4. Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic
veno-occlusive disease (sinusoidal obstruction syndrome) after
hematopoietic stem cell transplantation. Mayo Clin Proc. 2003;78:589-98.
5. Valla DC, Cazals-Hatem D. Sinusoidal obstruction
syndrome. Clin Res Hepatol Gastroenterol. 2016 Mar 30. pii:
S2210-7401(16)30034-1.
6. DeLeve LD, Valla DC, Garcia-Tsao G. Vascular
disorders of the liver. American Association for the Study Liver
Diseases. Hepatology. 2009;49: 1729-64.
7. Carreras E, Bertz H, Arcese W, Vernant JP, Tomás
JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive
disease after blood or marrow transplantation: a prospective cohort
study of the European Group for Blood and Marrow Transplantation.
European Group for Blood and Marrow Transplantation Chronic Leukemia
Working Party. Blood. 1998;92:3599-604.
8. Salat C, Holler E, Kolb HJ, Pihusch R, Reinhardt
B, Penovici M, et al. The relevance of plasminogen activator
inhibitor 1 (PAI-1) as a marker for the diagnosis of hepatic veno-occlusive
disease in patients after bone marrow transplantation. Leuk Lymphoma.
1999;33:25-32.
9. Nürnberger W, Michelmann I, Burdach S, Göbel U.
Endothelial dysfunction after bone marrow transplantation: increase of
soluble thrombomodulin and PAI-1 in patients with multiple
transplant-related complications. Ann Hematol. 1998;76:61-5.
10. Cutler C, Kim HT, Ayanian S, Bradwin G, Revta C,
Aldridge J, et al. Prediction of veno-occlusive disease using
biomarkers of endothelial injury. Biol Blood Marrow Transplant.
2010;16:1180-5.
11. McDonald GB, Sharma P, Matthews DE, Shulman HM,
Thomas ED. Venocclusive disease of the liver after bone marrow
transplantation: diagnosis, incidence, and predisposing factors.
Hepatology. 1984;4:116-22.
12. Jones RJ, Lee KS, Beschorner WE, Vogel VG,
Grochow LB, Braine HG, et al. Venoocclusive disease of the liver
following bone marrow transplantation. Transplantation. 1987;44:778-83.
13. Oakhill A, Green ID, Knowlson GT,Cameron AH, Shah
KJ, Hill FG, et al. Busulphan lung in childhood. J Clin Pathol.
1981;34:495.
14. Lund MB, Brinch L, Kongerud J, Boe J. Lung
function 5 yrs after allogeneic bone marrow transplantation conditioned
with busulphan and cyclophosphamide. Eur Respir J. 2004;23:901.
15. Vergnon JM, Boucheron S, Riffat J, Guy C, Blanc
P, Emonot A. Interstitial pneumopathies caused by busulfan. Histologic,
developmental and bronchoalveolar lavage analysis of 3 cases. Rev Med
Interne. 1988;9:377.
|
|
|
 |
|

