|
|
|
Indian Pediatr 2017;54: 835-840 |
 |
Impact of High-flow
Nasal Cannula Therapy in Quality Improvement and Clinical
Outcomes in a Non-invasive Ventilation Device-free Pediatric
Intensive Care Unit
|
|
Fulva Kamit Can, Ayse Berna Anil, Murat Anil,
Neslihan Zengin, Alkan Bal, Yuksel Bicilioglu, Gamze Gokalp, Fatih Durak
and Gulberat Ince
From Pediatric Intensive Care Unit, Izmir Tepecik
Training and Research Hospital, Turkey.
Correspondence to: Dr Fulya Kamit Can, Izmir Tepecik
Teaching and Research Hospital, Pediatric Intensive Care Unit, Yenisehir/
Konak, (35000) Izmir, Turkey.
Email: [email protected]
Received: September 06, 2016;
Initial Review: December 20, 2016;
Accepted:June 30, 2017.
Published online: July 11, 2017.
PII:S097475591600072
|
|
Objective: To analyze the change
in quality indicators due to the use of high-flow nasal cannula therapy
as a non-invasive ventilation method in children with respiratory
distress/failure in a non-invasive ventilation device-free pediatric
intensive care unit. Methods: Retrospective chart review of
children with respiratory distress/failure admitted 1 year before
(period before high-flow nasal cannula therapy) and 1 year after (period
after high-flow nasal cannula therapy) the introduction of high-flow
nasal cannula therapy. We compared quality indicators as rate of
mechanical ventilation, total duration of mechanical ventilation, rate
of re-intubation, pediatric intensive care unit length of stay, and
mortality rate between these periods. Results: Between November
2012 and November 2014, 272 patients: 141 before and 131 after high-flow
nasal cannula therapy were reviewed (median age was 20.5 mo). Of the
patients in the severe respiratory distress/failure subgroup, the rate
of intubation was significantly lower in period after than in period
before high-flow nasal cannula therapy group (58.1% vs. 76.1%;
P <0.05). The median pediatric intensive care unit length of stay
was significantly shorter in patients who did not require mechanical
ventilation in the period after than in the period before high-flow
nasal cannula therapy group (3d vs. 4d; P<0,05).
Conclusions: Implementation of high-flow nasal cannula therapy in
pediatric intensive care unit significantly improves the quality of
therapy and its outcomes.
Keywords: Endotracheal intubation, Mechanical
ventilation, Respiratory distress/failure.
|
|
|
|
H
igh-flow nasal cannula therapy (HFNC), is a
non-invasive form of oxygen delivery, wherein heated, humidified, and
blended oxygen/air reduces damage to the upper airway mucosa, increases
ciliary activity, decreases viscosity of secretions and may reduce
airway edema that makes it a comfortable way of oxygenation [1-4]. Most
studies on HFNC therapy have been performed in neonates or in the post-extubation
period in children or infants with bronchiolitis. They have reported
some advantages, such as easy application and tolerability [5-8]. A few
studies have been conducted in pediatric intensive care units (PICUs),
with most of these concentrating on bronchiolitis [9-12]. Studies
evaluating the effectiveness of HFNC in children with various etiologies
of respiratory distress between 1 month and 18 years of age in PICUs are
very limited [13-15].
The aim of our study was to analyze the effectiveness
of HFNC therapy as a non-invasive ventilation (NIV) method in quality
improvement and clinical outcomes of children aged 1 month to 18 years
with various etiologies of respiratory distress/failure in the PICU.
Methods
This study was a retrospective chart review of
children with respiratory distress/failure admitted 1 year before and 1
year after the introduction of HFNC therapy. Our PICU is in Tepecik
Teaching and Research Hospital in Ýzmir, Turkey. It is a 10-bed tertiary
mixed surgical and medical unit. There was only invasive mechanical
ventilation (MV) as a ventilation method in our PICU before the
implementation of HFNC. In this period, nasal cannula oxygen, hood,
simple face mask, and non-rebreather mask were used. HFNC therapy was
first used on November 1, 2013. After the introduction of HFNC, all
patients received HFNC as the primary respiratory support for
respiratory distress/failure according to our HFNC protocol. No other
non-invasive ventilation device was used in our PICU and there was no
HFNC in our Pediatric Emergency Department (PED) during the study
period. The nursing staff, the intensive care specialists, the standard
care, the admission criteria, the decision for intubation and the
respiratory support methods given other than HFNC were similar in these
periods.
In the protocol, the oxygen flow rates used were
5-50 L/min depending on the patient response (respiratory rate, heart
rate, SpO 2, perfusion,
comfort) with a FiO2 between
0.3 and 1. The inspired oxygen concentration was titrated to achieve SpO2
> 94%. SpO2/FiO2
ratio was used to determine the requirement for oxygen. The flow rate
was set at 5 L/min for infants or 15 L/min for children at the
beginning, and was titrated (±5 L/min) to achieve reduction of the
oxygen requirement to FiO2
of 30% and to improve the work of breathing, respiratory rate, and heart
rate. The flow rate used in infants was 5-20 L/min and for children,
15-50 L/min. HFNC was discontinued if there was clinical deterioration
(oxygen requirement, work of breathing, respiratory rate, or heart rate)
in the first 30 min and the patients were intubated and ventilated
mechanically. If there was no change in the first 30 min, the patients
were followed for 30 min longer. The HFNC system (Fisher & Paykel
Healthcare Airvo 2) comprises a humidifier (MR290) and a continuous flow
circuit (900PT531 for infants, 900PT501 for children). We selected the
nasal prong size that best fitted the nostrils (Optiflow, OPT318,
OPT842, OPT844, OPT846).
We evaluated whether the clinical outcomes improved
due to using HFNC therapy. We chose five quality measurements that
indicate success, failure or ineffectiveness: the rate of MV,
total duration of MV, rate of re-intubation, PICU length of stay (LOS)
and rate of mortality. The patients with respiratory distress or failure
between 1 month to 18 years of age who stayed more than 24 h in the PICU
were included to the study. The definition of "respiratory distress" was
hypoxemia (SpO 2<94%),
tachypnea, increased work in breathing (chest wall retraction, use of
accessory respiratory muscles, nasal flaring/grunting, feeding
difficulties). Poor perfusion (cyanosis, mottling, poor neurological
status, reduced muscle tonus), apnea or PaO2
< 50 mmHg in room air, respiratory acidosis
(pH<7.35), PaO2<60 mmHg when
FiO2 60%, and PaCO
> 60 mmHg in arterial blood gas analysis were deemed "respiratory
failure". The patients admitted between November 1, 2012 and November 1,
2014 to our PICU were evaluated. Thus, 1 year before HFNC therapy was
defined as the period before HFNC and 1 year after the introduction of
HFNC therapy was defined as the period after HFNC. Patients were
excluded if they had had a tracheostomy, if they were intubated before
PICU admission, or if they stayed less than 24 h in the PICU. We
estimated the severity of respiratory distress by using a score, which
can be used for a large range of ages and etiologies of respiratory
distress [16]. Pediatric index of mortality 2 (PIM 2) and pediatric risk
of mortality (PRISM) scores were routinely used in the PICU.
Calculations of these scores were made using web-based calculators (http://www.sfar.org/article/316/scoring-systems-for-icu-and-surgical-patients).
Primary diagnosis of patients were categorized
(intoxication, sepsis, trauma, post-op, neurological, respiratory,
gastrointestinal, metabolic, hemato- oncological and cardiovascular) and
analyzed. We classified the patients into seven categories according to
etiology of their respiratory distress/failure: bronchiolitis,
pneumonia, upper airway obstruction, extra pulmonary acute lung injury
(ALI), asthma, neuromuscular diseases, and other pulmonary diseases. We
divided the patients in to two groups according to the severity of
respiratory distress/failure: patients with mild-moderate and severe
respiratory distress. The demographic and clinical parameters (body
weight, mortality scores, presence of chronic disease, primary diagnose,
etiology of respiratory distress/ failure, severity groups, rate of MV,
total duration of MV, rate of re-intubation, PICU LOS and rate of
mortality) were compared between the two time-periods. We also analyzed
the subgroups: mild-moderate respiratory distress group, severe
respiratory distress group, patients with respiratory distress/failure
due to pulmonary disease (bronchiolitis, pneumonia, upper airway
obstruction, other pulmonary diseases and asthma), patients diagnosed as
bronchiolitis, patients required MV, and patients who did not required
MV. The study was approved by the local ethics committee.
The study databases were analyzed using the SPSS
software (ver. 20.0; SPSS Inc., Chicago, IL). Numerical variables were
analyzed using the Mann-Whitney U-test. Categorical variables were
compared using the Chi-square or Fisher’s exact test, as appropriate.
Differences were considered significant at P < 0.05.
Results
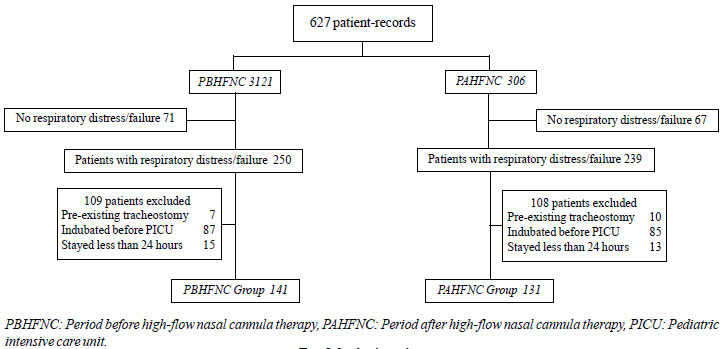
In total, 272 records (141 in the before the
high-flow nasal cannula introdution) were reviewed (Fig 1).
In study group, 137 (50.3%) patients were intubated (72 in the before
group, 65 in the after high flow nasal cannula group) and 46 (16.9%)
patients died. There was no difference in the rate of mechanical
ventilation or its duration, rate of re-intubation, PICU LOS, or
mortality rate between the groups (Table I). We found no
difference in the primary diagnosis (diagnostic categories of patients
at admission) or etiology of respiratory distress/failure between the
intubated patients in the two groups.
 |
|
Fig. 1 Study algorithm
|
TABLE I The Comparison of Demographic Features, Clinical Parameters, and Outcomes between the two Groups.
|
Characteristic(median / |
Total (n=272) |
PBHFNC (n= 141) |
PAHFNC (n= 131) |
P |
|
interquartile range or n, %) |
|
|
|
|
|
Age in months |
20.5 (5- 75) |
22 (4.5- 49) |
18 (5- 70) |
0.414 |
|
Sex (n, %), female |
116 (42.6) |
55 (39) |
61 (46.5) |
0.183 |
|
Weight, kg |
10 (6- 16) |
10 (5- 15) |
13 (7- 16) |
0.051 |
|
PIM2 (%) |
21.8 (8-45) |
23.2 (8-45) |
20.4 (8-42) |
|
|
PRISM (%) |
32.3 (12-60) |
31.3 (12-60) |
33.4 (12-58) |
0.382 |
|
Chronic disease (+) |
154 (56.6) |
77 (54.6) |
77 (58.8) |
0.488 |
|
Primary diagnosis at admission |
|
|
|
|
|
Intoxication |
5 (1.8) |
3 (2.1) |
2 (1.5) |
|
|
Sepsis |
89 (32.7) |
49 (34.8) |
40 (30.5) |
|
|
Trauma |
9 (3.3) |
4 (2.8) |
5 (3.8) |
|
|
Post-op |
12 (4.4) |
5 (3.5) |
7 (5.3) |
0.877 |
|
Neurological |
5 (1.8) |
2 (1.4) |
3 (2.3) |
|
|
Respiratory |
121 (44.5) |
63 (44.7) |
58 (44.3) |
|
|
Gastrointestinal |
1 (0.4) |
0 |
1 (0.8) |
|
|
Metabolic |
11 (4) |
7 (5) |
4 (3.1) |
|
|
Hemato-oncological |
4 (1.5) |
1 (0.7) |
3 (2.3) |
|
|
Cardiovascular |
15 (5.5) |
7 (5) |
8 (6.1) |
|
|
Etiologies for respiratory distress/ failure |
|
|
|
|
|
Bronchiolitis |
41 (15.1) |
23 (16.3) |
18 (13.7) |
|
|
Pneumonia |
69 (25.4) |
35 (24.8) |
34 (26) |
|
|
Upper airway obstruction |
12 (4.4) |
6 (4.3) |
6 (4.6) |
|
|
Other pulmonary diseases |
41 (15.1) |
23 (16.3) |
18 (13.7) |
|
|
Extrapulmonary ALI/ARDS |
80 (29.4) |
45 (31.9) |
35 (26.7) |
|
|
Neuromuscular disease |
22 (8.1) |
6 (4.3) |
16 (12.2) |
|
|
Asthma |
7 (2.6) |
3 (2.1) |
4 (3.1) |
0.338 |
|
Severity of respiratory distress |
|
|
|
|
|
Mild |
16 (5.9) |
10 (7.1) |
6 (4.6) |
|
|
Moderate |
70 (25.7) |
43 (30.5) |
27 (20.6) |
0.089 |
|
Severe |
186 (68.4) |
88 (62.4) |
98 (74.8) |
|
|
MV (+) |
137 (50.4) |
72 (51.1) |
65 (50) |
0.861 |
|
Duration of MV (d) |
4 (1-11) |
4 (1-11) |
4 (1-11) |
0.440 |
|
Re-intubation (+) |
32 (11.8) |
15 (21.1) |
17 (27.4) |
0.397 |
|
LOS in PICU (d) |
8 (2-20) |
8.5 (2-18) |
7 (3-23) |
0.079 |
|
Death (+) |
46 (16.9) |
25 (17.7) |
21 (16) |
0.709 |
|
PBHFNC: Period before high-flow nasal cannula, PAHFNC: Period
after high-flow nasal cannula. ALI: Acute lung injury, ARDS:
Acute respiratory distress syndrome. PIM2: Pediatric index of
mortality 2, PRISM: Pediatric risk of mortality. MV: Mechanical
ventilation, LOS in PICU: Length of stay in pediatric intensive
care unit.
|
Of the severe respiratory distress group, the rate of
intubation was significantly lower in the after high flow nasal cannula
group (58.1% vs. 76.1%; P< 0.05) (Web Table I).
In total, 137 (50.8%) patients required mechanical
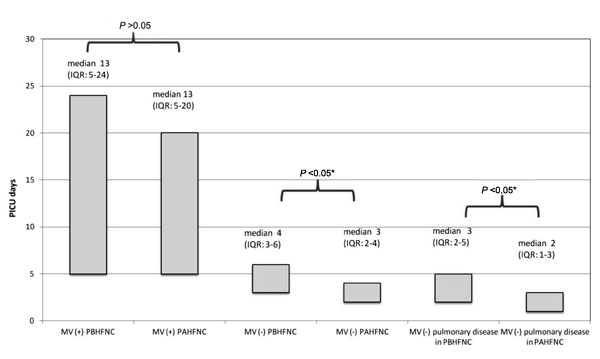
ventilation. The median PICU LOS was not significantly different in the
patients who required mechanical ventilation between the before (median:
13 days; IQR: 5-24) and after high-flow nasal cannula (median: 13 days;
IQR: 5-20) groups (P=0.7). Among the patients who did not require
mechanical ventilation (134; 49.2%), the median PICU LOS in after high
flow nasal cannula group was significantly shorter [median (IQR) 3 (2-4)
d vs. 4 (3-6) d; P=0.018]. When we analyzed the patients
with pulmonary disease who did not require mechanical ventilation, the
median PICU LOS was also shorter in the former [median (IQR) 2(1-3) d
vs. 3 (2-5) d; P=0.005] (Fig. 2).
MV: Mechanical ventilation, PICU: Pediatric
intensive care unit, PBHFNC: Period before high-flow nasal
cannula, PAHFNC: Period after high-flow nasal cannula, *statistically
significant.
|
|
Fig. 2 Comparison of length of PICU
stay between patients with and without MV support in the PBHFNC
and PAHFNC groups.
|
Discussion
In this retrospective chart review, we found that the
use of HFNC therapy reduced rate of mechanical ventilation in the
children with severe respiratory distress/failure subgroup. Furthermore,
we found that the use of HFNC as a primary support decreased the PICU
LOS in children who did not require intubation or mechanical
ventilation.
Our study had several limitations; the most important
being inadequate sample size. This makes it difficult to analyze the
effectiveness of HFNC for specific diseases. The heterogeneity of the
age of the study population was also a limiting factor. Another limiting
factor was that the study was conducted retrospectively in a single
center. We could not evaluate more changes in vital parameters, changes
in laboratory findings, or patient comfort levels after initiating HFNC
therapy, because of the retrospective nature of the study.
As in our study, Wing, et al. [17] found that
the need for intubation and the use of mechanical ventilation in the
PICU were decreased after the implementation of a hospital-wide
guideline for HFNC use. Nonetheless, they found no difference in the
PICU LOS, mortality rate, or duration of mechanical ventilation. HFNC
was shown to be a safe and an effective form of respiratory support in
the PICU, and the prognosis of patients could be predicted by simple
bedside observations [13]. However, a meta- analysis determined no
evidence for the safety or effectiveness of HFNC as a form of
respiratory support in children [18]. In the literature, reduced
intubation rates in patients with bronchiolitis have been reported after
the use of HFNC [7,8]. However, in a meta-analysis, it was determined
that there was insufficient evidence for the effectiveness of HFNC
therapy for infants with bronchiolitis [19]. In our study, patients with
bronchiolitis constituted 15% of all patients, and we did not find any
significant impact on clinical outcomes in patients with bronchiolitis.
In conclusion, we showed that the implementation of
HFNC therapy improved clinical outcomes in children aged 1 month to 18
years with various etiologies of severe respiratory distress/failure
admitted to the non-invasive ventilation device-free PICU. To better
understand the effectiveness of HFNC in the PICU, prospective
randomized-controlled studies are needed.
Contributors: FKC: study design, analysis of
data, manuscript preparation; ABA, MA: study design, analysis of data,
review of manuscript; NZ, AB, YB, GG, FD, GI: literature search, data
collection.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
•
Implementation of high-flow
nasal cannula therapy improved the quality of treatment and its
clinical outcomes in children with respiratory distress/failure
in non-invasive ventilation device-free PICU.
|
References
1. Ward JJ. High-flow oxygen administration by nasal
cannula for adult and perinatal patients. Respir Care. 2013;58:98-120.
2. Dysart K, Miller TL, Wolfson MR, Shaffer TH.
Research in high flow therapy: Mechanisms of action. Respir Med.
2009;103:1400-05.
3. Spence KL, Murphy D, Kilian C, McGonigle R, Kilani
RA. High-flow nasal cannula as a device to provide continuous positive
airway pressure in infants. J Perinatol. 2007;27:772-5.
4. Urbano J, del Castillo J, Lo´pez-Herce J, Gallardo
JA, Solana MJ, Carrillo A. High-flow oxygen therapy: Pressure analysis
in a pediatric airway model. Respir Care. 2012;57:721-6.
5. Ojha S, Gridley E, Dorling J. Use of heated
humidified high-flow nasal cannula oxygen in neonates: A UK wide survey.
Acta Paediatrica. 2013;102:249-53.
6. Hough JL, Shearman AD, Jardine LA, Davies MW.
Humidified high flow nasal cannulae: Current practice in Australasian
nurseries, a survey. J Paediatr Child Health. 2012;48:106-13.
7. McKiernan C, Chua LC, Visintainer PF, Allen H.
High flow nasal cannulae therapy in infants with bronchiolitis. J
Pediatr. 2010;156:634-8.
8. Schibler A, Pham TMT, Dunster KR, Foster K, Barlow
A, Gibbons K, et al. Reduced intubation rates for infants after
introduction of high-flow nasal prong oxygen delivery. Intensive Care
Med. 2011;37:847-52.
9. Metge P, Grimaldi C, Hassid S, Thomachot L,
Loundou A, Martin C, et al. Comparison of a high-flow humidified
nasal cannula to nasal continuous positive airway pressure in children
with acute bronchiolitis: Experience in a pediatric intensive care unit.
Eur J Pediatr. 2014;173: 953-8.
10. Campaña MB, Ortiz JO, Muñoz CN, Lucas MR, Rincón
AF, Hernández OP, et al. High flow therapy versus hypertonic
saline in bronchiolitis: Randomised controlled trial. Arch Dis Child.
2014;99:511-5.
11. Milesi C, Baleine J, Matecki S, Durand S, Combes
C, Novais ARB, et al. Is treatment with a high flow nasal cannula
effective in acute viral bronchiolitis? A physiologic study. Intensive
Care Med. 2013;39:1088-94.
12. Abboud PA, Roth PJ, Skiles CL, Stolfi A, Rowin
ME. Predictors of failure in infants with viral bronchiolitis treated
with high-flow, high-humidity nasal cannula therapy. Pediatr Crit Care
Med. 2012;13:343-49.
13. Brink F, Duke T, Evans J. High-flow nasal prong
oxygen therapy or nasopharyngeal continuous positive airway pressure for
children with moderate-to-severe respiratory distress? Pediatr Crit Care
Med. 2013;14:326-31.
14. Mayfield S, Jauncey-Cooke J, Bogossian F. A case
series of paediatric high flow nasal cannula therapy. Aust Crit Care.
2013;26:189-92.
15. García Figueruelo A, Urbano Villaescusa J, Botrán
Prieto M, Solana García MJ, Mencía Bartolomé S, López-Herce Cid J. Use
of high-flow nasal cannula for non-invasive ventilation in children. Ann
Pediatr (Barc). 2011;75:182-7.
16. Liu LL, Gallaher MM, Davis RL, Rutter CM, Lewis
TC, Marcuse EK. Use of a respiratory clinical score among different
providers. Pediatr Pulmonol. 2004;37:243-8.
17. Wing R, James C, Maranda LS, Armsby CC. Use of
high-flow nasal cannula support in the emergency department reduces the
need for intubation in pediatric acute respiratory insufficiency.
Pediatric Emergency Care. 2012;28:1117-23.
18. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler
A, Gibbons K, Bogossian F. High flow nasal cannula therapy for
respiratory support in children. Cochrane Database Syst Rev.
2014;7;3:CD009850.
19. Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA.
High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane
Database Syst Rev. 2014;20;1:CD009609.
|
|
|
 |
|

