Pediatric tuberculosis (i.e., Tuberculosis (TB)
among the population aged less than 14 years) has traditionally received
a lower priority than adult TB in National TB programmes because of its
considered non-infectious, is difficult to diagnose, cases have been
thought to be few and it was wrongly assumed that effective control of
adult TB and use of BCG by itself could prevent childhood TB. Contrary
to traditional National TB programmes, pediatric tuberculosis (i.e.,
TB among the population aged less than 14 years) has always been
accorded high priority by Revised National Tuberculosis Control
Programme (RNTCP) since the inception of the programme in our country.
In India, there are about ~400 million children who
constitute about 34% of the total population [1]. The extent of
childhood TB in India is unknown due to diagnostic difficulties; it is
estimated to be 10.2% of the total adult incidence [2]. The maximum risk
of a child getting TB is between 1-4 years when there is an increased
risk of progression from infection to disease. Globally, about 1 million
cases of pediatric TB are estimated to occur every year accounting for
10-15% of all TB [3]; with more than 100,000 estimated deaths every
year, it is one of the top 10 causes of childhood mortality. Though
MDR-TB and XDR-TB is documented among pediatric age group, there are no
estimates of overall burden, chiefly because of diagnostic difficulties
and exclusion of children in most of the drug resistance surveys.
The proportion of pediatric TB cases registered under
RNTCP has shown an increasing trend, from 5.6% (59846 cases) in 2005 to
7% (84064 cases) in 2011 [4]. RNTCP in association with Indian Academy
of Pediatrics (IAP) has described criteria for suspecting TB among
children; has separate algorithms for diagnosing pulmonary TB and
peripheral TB lymphadenitis and a strategy for treatment and monitoring
patients who are on treatment. In brief, TB diagnosis is based on
clinical features, smear examination of sputum where this is available,
positive family history, tuberculin skin testing, chest radiography and
histopathological examination as appropriate. As in adults, children
with TB are classified, categorised, registered and treated with
intermittent short-course chemotherapy (thrice-weekly therapy from
treatment initiation to completion), given under direct observation of a
treatment provider (DOT provider) and the disease status is monitored
during the course of treatment. Based on their pre- treatment weight,
children are assigned to one of pre-treatment weight bands and are
treated with good quality anti-TB drugs through ‘‘ready-to-use’’ patient
wise boxes containing the patients’ complete course of anti-TB drugs are
made available to every registered TB patient according to programme
guidelines. India was the first country to introduce pediatric
patient-wise boxes.
2. National Consultation on Diagnosis and Management of Childhood
Tuberculosis [5]
In order to reconcile between Global and National
guidelines, to review the evidence base and update the RNTCP guidelines
in consensus with Indian academy of paediatrics, a National consultation
was organized in January 2012. The consultation has come up with wider
recommendations that have been incorporated in the programme.
 |
|
Fig.1a Diagnostic algorithm for
pediatric pulmonary tuberculosis
|
2.1 Diagnosis of pediatric TB: A new
diagnostic algorithm is developed for pulmonary TB, the commonest type
of extra pulmonary TB (Lymph node TB) and for other types of
extra-pulmonary TB. The diagnostic algorithms for the diagnosis of
pulmonary TB and Lymph node tuberculosis are provided in Fig.
1. The salient recommendations are:
(a) All efforts should be made to
demonstrate bacteriological evidence for the diagnosis of pediatric
TB. In cases where sputum is not available for examination or sputum
microscopy fails to demonstrate AFB, alternative specimens (Gastric
lavage, Induced sputum, broncho-alveolar lavage) should be
collected, depending upon the feasibility, under the supervision of
a pediatrician.
(b) A positive Tuberculin skin test/Mantoux
test was defined as an induration of 10 mm or more, measured 48-72
hours after Intradermal injection with Tuberculin 2 TU (RT 23 or
equivalent). In HIV cases the cut off is reduced to 5 mm or more of
induration.
(c) There is no role for
inaccurate/inconsistent diagnostics like serology (IgM, IgG, IgA
antibodies against MTB antigens), various in-house or non-validated
commercial PCR tests and BCG test.
(d) There is no role of IGRAs in clinical
practice for the diagnosis of TB.
(e) Loss of weight – often used as a
clinical marker for the disease has been objectively defined as a
loss of more than 5% of the highest weight recorded in the past
three months.
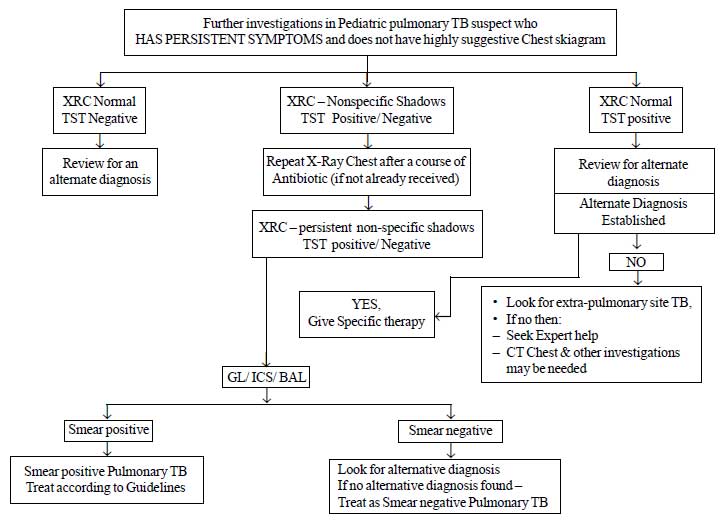 |
|
Fig.1b Diagnostic algorithm for
pediatric pulmonary tuberculosis.
|
2.2 Intermittent versus Daily regimen: The
intermittent therapy will remain the mainstay of treating pediatric
patients. However, among seriously ill admitted children or those with
severe disseminated disease/ neuro-tuberculosis, the likelihood of
vomiting or non-tolerance of oral drugs is high in the initial phase.
Such, select group of seriously ill admitted patients can be given daily
supervised therapy during their stay in the hospital using
daily drug dosages. After discharge they will be taken on thrice weekly
DOT regimen (with suitable modification to thrice weekly dosages). The
following are the daily doses (mg per kg of body weight per day)
Rifampicin 10-12 mg/kg (max 600 mg/day), Isoniazid 10 mg/kg (max 300
mg/day), Ethambutol 20-25 mg/kg (max 1500 mg/day), PZA 30-35 mg/kg (max
2000 mg/day) and Streptomycin 15 mg/kg (max 1g/day).
2.3 The following newer Case
definitions for pediatric TB patients will be incorporated in the
RNTCP manuals:
(a) Failure to respond: A case of
pediatric TB who fails to have bacteriological conversion to
negative status or fails to respond clinically/or deteriorates after
12 weeks of compliant intensive phase shall be deemed to have failed
response provided alternative diagnoses/reasons for nonresponse have
been ruled out.
(b) Relapse: A case of pediatric TB
declared cured/completed therapy in past and has (clinical or
bacteriological) evidence of recurrence.
(c) Treatment after default: A case
of pediatric TB who has taken treatment for at least 4 weeks and
comes after interruption of treatment for 2 months or more and has
active disease (clinical or bacteriological).
For programmatic purposes of reporting, all types of
retreatment cases where bacteriological evidence could not be
demonstrated but decision to treat again was taken on clinical grounds
would continue to be recorded and reported as "Others" for
surveillance purposes.
2.4 Drug dosages:
(a) To meet the pediatric fraternity
concerns about under dosing and also in view of the latest WHO
guidance, the drug dosages have been rationalized for childhood
cases. There shall be six weight bands
(6-8,9-12,13-16,17-20,21-24,and 25-30 kg) and the existing pediatric
PWBs are to be used in different combinations to meet these
expectations. In future, three generic patient wise boxes (instead
of the existing two) will be used in combination to treat patients
in these six weight bands. It would take at-least 2 years for supply
of these new products under RNTCP.
(b) To ensure that every child gets
correct dosages, weighing of the patient in minimal clothing (as
appropriate) using accurate weighing scales is essential.
(c) It was also agreed that, all pediatric
TB patients should be shifted to next weight band if a child gains a
kilogram or more, above the upper limit of the existing weight band.
2.5 Drug formulations: Since, the number
of tablets is too many to consume and younger patients have difficulty
in swallowing tablets the DOT centers will be provided with pestle
and mortars for crushing the drugs. It will be the responsibility of
the DOT provider to supervise the process of drug consumption by the
child and in case any child vomits within half an hour of period of
observation, fresh dosages for all the drugs vomited will be provided to
the caregiver. The programme will continue to explore the possibility of
using quality fixed dose combinations and dispersible tablets in future.
2.6 Treatment regimens: There will be only
two treatment categories – one for treating ‘new’ cases and another for
treating ‘previously treated cases.’ (Table I) Three drug
category III regime has been since withdrawn in view of high INH
resistance (>5%) in our community.
TABLE I Treatment Categories and Regimens for Childhood Tuberculosis
|
Category of treatment
|
Type of patients |
TB treatment regimens
|
|
|
Intensive
|
Continuation
|
|
|
phase |
phase |
|
New cases |
• New smear-positive pulmonary |
2H3R3Z3E3* |
4H3R3
|
|
Tuberculosis (PTB) |
|
|
|
• New smear-negative PTB |
|
|
|
• New extra-pulmonary TB |
|
|
|
Previously treated cases |
• Relapse, failure to respond or treatment
after default |
2S3H3R3Z3E3
+ 1H3R3Z3E3 |
5H3R3E3 |
|
• Re-treatment Others |
|
|
H=Isoniazid, R= Rifampicin, Z= Pyrazinamide, E= Ethambutol,
S= Streptomycin. *The number before the letters refers to the
number of months of treatment. The subscript after the letters
refers to the number of doses per week.Pulmonary TB refers to
disease involving lung parenchyma. Extra Pulmonary TB refers to
disease involving sites other than lung parenchyma. If both
pulmonary and extra pulmonary sites are affected, it will be
considered as Pulmonary for registration purposes. Extra
Pulmonary TB involving several sites should be defined by most
severe site.
Smear positive: Any sample
(sputum, induced sputum, gastric lavage, broncho-alveolar lavage)
positive for acid fast bacilli.New Case: A patient who has had
no previous ATT or for less than 4 weeks.
Relapse: Patient declared
cured/completed therapy in past and has evidence of recurrence.
Treatment after Default: A patient who has taken treatment for
at least 4 weeks and comes after interruption of treatment for 2
months and has active disease.
Failure to respond: A case of
pediatric TB who fails to have bacteriological conversion to
negative status or fails to respond clinically or deteriorates
after 12 weeks of compliant intensive phase shall be deemed to
have failed response, provided alternative diagnoses/ reasons
for non-response have been ruled out.
Others: Cases who are smear
negative or extra pulmonary but considered to have relapse,
failure to respond or treatment after default or any other case
which do not fit the above definitions.
In patients with TB meningitis on Category I treatment, the four
drugs used during the intensive phase can either be HRZE or
HRZS. The present evidence suggests that Ethambutol should be
preferred in children.Children who show poor or no response at 8
weeks of intensive phase may be given benefit of extension of IP
for one more month. In patients with TB Meningitis, spinal TB,
miliary/disseminated TB and osteo-articular TB, the continuation
phase shall be extended by 3 months making the total duration of
treatment to a total of 9 months. A further extension may be
done for 3 more months in continuation phase (making the total
duration of treatment to 12 months) on a case to case basis in
case of delayed response and as per the discretion of the
treating physician.Under Revised National Tuberculosis Program
(RNTCP, all patients shall be covered under directly observed
intermittent (thrice weekly) therapy. The supervised therapy is
considered as the most optimal treatment and is followed under
RNTCP. It is important to ensure completion of treatment
in every case put on treatment to prevent emergence of
resistance, particularly to Rifampicin. In the rare
circumstances where a patient is given daily therapy,
observation and completion of therapy remains as important. It
is the duty of the prescriber to ensure appropriate and complete
treatment in all cases. |
2.7 TB Meningitis: In the management of TB
Meningitis, the group recommended that streptomycin can be safely
replaced by ethambutol in intensive phase of TBM because of (a)
current evidence favoring safety and efficacy of ethambutol, (b)
lack of any value addition in efficacy using Streptomycin over
ethambutol, and (c) need to avoid problems of injection based
treatment (lack of adequate muscle mass in malnourished, risks of unsafe
Injections, need for a trained personnel, unpleasantness of the
treatment). While ethambutol was considered a better option to replace
streptomycin in the treatment of new cases of childhood TB, streptomycin
continues to be recommended as the additional fifth drug in the
retreatment regime.
2.8 Extending intensive and continuation
phase:
(a) Children who show inadequate or no
response (on smear or clinico-radiological basis) at 8 weeks of
intensive phase should be given benefit of extension of IP for one
more month.
(b) In patients with TB Meningitis, spinal
TB, miliary/disseminated TB and osteo-articular TB, the continuation
phase shall be extended by 3 months making the total duration of
treatment to a total of 9 months. A further extension may be done
for 3 more months in continuation phase (making the total duration
of treatment to 12 months) on a case to case basis in case of
delayed response and as per the discretion of the treating
physician/ pediatrician.
2.9 TB preventive therapy: The currently
recommended dose of INH for chemoprophylaxis is 10 mg/kg (instead of
currently recommended dosage of 5 mg/kg) administered daily for 6
months. TB preventive therapy should be provided to:
(a) All asymptomatic contacts (under 6
years of age) of a smear positive case, after ruling out active
disease and irrespective of their BCG, TST or nutritional status.
(b) Chemoprophylaxis is also recommended
for all HIV infected children who either had a known exposure to an
infectious TB case or are Tuberculin skin test (TST) positive (>=5
mm induration) but have no active TB disease.
(c) All TST positive children who are
receiving immunosuppressive therapy (e.g. Children with
nephrotic syndrome, acute leukemia, etc.).
(d) A child born to mother who was
diagnosed to have TB in pregnancy should receive prophylaxis for 6
months, provided congenital TB has been ruled out. BCG vaccination
can be given at birth even if INH chemoprophylaxis is planned.
3. Way Forward
These consensus National Guidelines on pediatric
tuberculosis was jointly developed in consultation with Indian Academy
of Pediatric and TB experts from various premier institutions in India.
Keeping the interests of the Nation at large, it is urged that all the
clinicians, teachers, academicians, researchers or any other person
dealing with pediatric tuberculosis with in the Government or Private or
non-governmental sector should adopt these guidelines for the diagnosis
and treatment of pediatric tuberculosis in India.

