|
AUTOIMMUNE THYROID DISEASE IN CHILDHOOD: A STUDY OF CHILDREN AND THEIR FAMILIES |
Meena P. Desai and Swati Karandikar
From SirHurkisondas Nurrotumdas Medical Research Society and Bai Jerbai Wadia Hospital for Children and Institute of Child Health and Research Center, Mumbai, India.
Reprint requests: Dr. Meena P. Desai, 307, Samudra Mahal, Worli, Mumbai 400 018, India.
Manuscript received: June 8, 1998; Initial review completed: July 27, 1998;
Revision accepted: March 1, 1999.
Abstract:
Objective: To study the clinical and laboratory profile of children with autoimmune thyroid disease (AITD) and its familial prevalence. Design: Clinical and investigative evaluation of96 children and adolescents 5 to 16 years old suspected of having AITD based on clinical and family data and similar assessment of parents and siblings of 30 confirmed cases of AITD. Setting and Subjects: Of these 96 cases, 66 were from a private clinic and 30 were institution based thyroid antibody positive with confirmed AlTD. On initial testing 36 (55%) of 66 clinic cases were thyroid antibodies (ab) positive and 30 were (ab) negative. In 12
of 30 above cases retesting for antibodies by newer technique or FNAC confirmed AITD. Clinical and laboratory evaluation of 90 of a total of 106 parents and siblings of the 30 institution based cases. Methods: Clinical evaluation with goiter grading by WHO criteria was done in all. Family history of thyroid disease was
inquired for in all. Clinical examination and thyroid antibody status was assessed in 90 family members as stated above. Thyroid antimicrosomal (AMA) and antithyroglobulin (ATG) antibodies were tested by standard hemogglutination kits. Titers of> 1:100 considered +ve for children and >1:400 for adults. Thyroid (ab) could be tested in ten of the
above cases by ECI technique on follow up. Bone age was assessed. Ultrasonographic or TCM 99 scanning of thyroid gland and FNA C were done as indicated. Results: Of the 96 children suspected to have AlTD, thyroid antibodies were
positive in
high
titers in
66 (36+30) cases (69%) on initial testing but with more sensitive ECl technique
significant antibody titres were detected in 10 more cases (79%) and FNAC confirmed AITD in 2 more subjects (total 78 - initial 66 + 12). F:M ratio was 2.9:1. Sixty one per cent of children were between 6 to 12 years of age; mean age 10.12:t 2.9 years. Seventy seven per cent had hypothyroidism, 10% had thyrotoxicosis and only 13% were euthyroid. Family history of thyroid disease was elicited in 33% of the series. Survey of90 parents and siblings of the institution based group revealed, euthyroid goiters in 17%, subclinical hypothyroidism in 10% and significant AMA titers in 43% (65% of mothers, 30% siblings and 43% fathers). Conclusion: Juvenile AlTD is a common cause of acquired thyroid disease in children above 5 years of age with a 3-fold higher prevalence in girls. The manifestations are heterogeneous. Hypothyroidism was most common (77%), euthyroid goiters (13%) and thyrotoxicosis (10%) were less
frequent. Familial aggregation was noted in adult family members (33%) with positive thyroid antibodies in 65% of mothers. Sibling affection was .less frequent. The familial and genetic implications of AITD are important; diagnosis of AlTD in children may also help detect subclinical disease in adult family members.
Key words: Autoimmune thyroid disease, Goiter, Thyroid antibodies.
AUTOIMMUNE thyroid disorders are no longer considered uncommon in children. Chronic thyroiditis or chronic lymphocytic thyroiditis has now emerged as one of the
leading causes of thyroid disorders in child- hood and adolescence and is considered the most common cause of acquired hypothyroid- ism in iodine sufficient regions(1). These immunologically mediated thyroid disorders have a genetic predisposition and a tendency to cluster in families(2). The clinical manifestations range from euthyroid goiter to hypo and hyperthyroid states. The spectrum of autoimmune thyroid disease (AlTO) includes a large number of clinical entities like Hashimoto's or chronic lymphocytic
thyroiditis (CLT), focal thyroiditis, fibrous thyroiditis, primary myxedema, Grave's disease and Hashitoxicosis(2,3). Simple adolescent and multinodular goiter, or rarely sporadic cretinism as well as the two transient neonatal syndromes of hypothyroidism and thryotoxicosis due to transplacental transfer of maternal thyroid antibodies also have an autoimmune basis(2,3). Isolated involvement of the thyroid gland is more common but occasionally occurs in association with other endocrinopathies and nonendocrine autoimmune disorders(3).
Information on AlTO in Indian children as well as its familial prevalence is not adequately documented. In our previous study on hypothyroid children (birth to 16 years) thyroiditis was the underlying cause in 5%(4). The objective of the present study was to obtain data on clinical and laboratory profile of children with AITD and study the familial prevalence of thyroid disease.
Subjects and Methods
Initial evaluation included 96 children and adolescents with goiter or thyroid dysfunction where the possibility of AITD was suspected clinically. In 66 cases thyroid antibody positivity confirmed the diagnosis at the outset whereas repeat studies confirmed AITD in 12 more cases on follow up.
The 96 children and adolescents examined
were between 5 to 16 years of age (69 girls and 27 boys). Sixty six of these children where AITD was suspected clinically, were from a private clinic of one of us whereas the rest 30 subjects were confirmed cases of AITD. Complete clinical and laboratory survey of the parents and siblings (first degree relatives) of these 30 cases was also conducted. Clinical suspicion of AITD was based on late onset of symptoms
above
4 to 5 years of age, normal
progress and development during infancy and early childhood, history of familial prevalence of thyroid disorders, presence of a diffuse firm thyromegaly of relatively recent onset with or without clinical signs of thyroid dysfunction, and associated clinical conditions or endocrinopathies favoring autoimmunity. Majority of these children were referred from the city or from areas of the state not specifically endemic for iodine deficiency.
Detailed history regarding the duration and nature of presenting complains was obtained. All details of developmental milestones and physical growth through early childhood and relevant past history were inquired for. Information on familial prevalence of thyroid
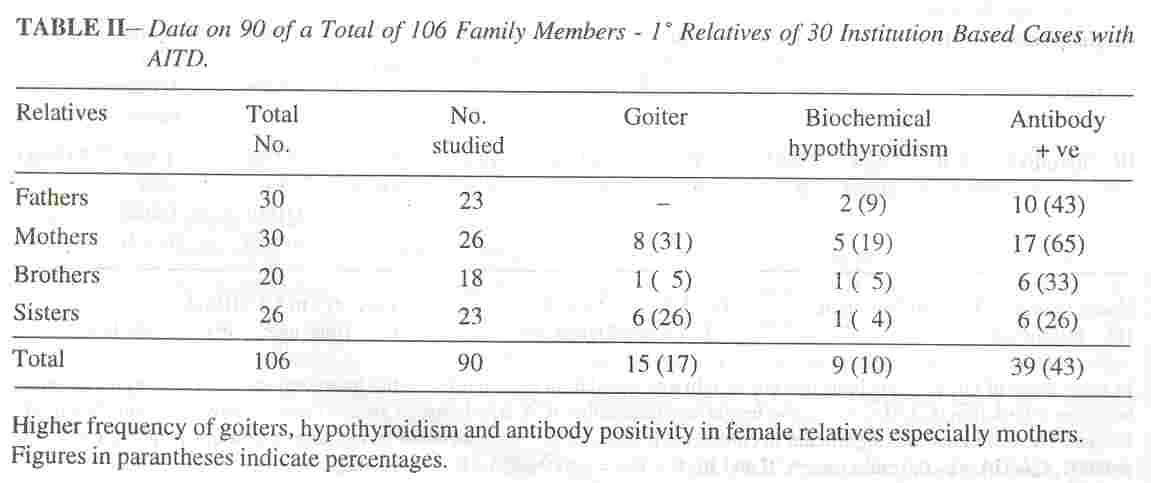
disorders was inquired for in all but complete clinical and laboratory evaluation of parents and siblings was restricted to the families of the 30 hospital based. children. Of a total of 106 first degree relatives of these 30 proven cases of AITD,.90 subjects were available for clinical and laboratory studies.
Careful clinical examination was carried out in all the patients including height and weight measurements. WHO grading system was used for grading goiter size; Grade la
-
enlarged gland not visible but on palpation size of the lobes larger than terminal phalanx of patient's thumb; Grade Ib
-
visible and
palpable gland with neck extended; Grade II - visible and palpable gland with neck in normal flexed
positition and Grade III - large gland
evident from a distance. Tanner's chart were used for grading heights and weights and for estimating the height age and sexual maturity. Bone age was assessed according to Greulich
and Pyle's atlas.
The thyroid homones, T3 and T4, were estimated by standard RIA kits, the sensitivity of the assay being 0.24 ng/ml and 0.5 J.l,g/dl, respectively. Serum TSH was assayed by immunoradiometric(IRMA) assay with a sensitivity of 0.07 J.l,U/ml and the laboratory normal range was 0.2 to 5.1 uU/mI. Antithyroglobulin (A TG) and antimicrosomal (AMA) [also known as Antiperoxidase (APO)] antibodies were tested by serodia kits macro- hemagglutination test with appropriate controls. Titers of 1 in 100 were. considered positive for ATA and AMA in children and 1in 400 and above for adult family members. During follow up, nearly 6 to 12 months later, thyroid antibodies could be restested in ten of the antibody negative cases by more recent techniques, Immulin Anti-TG and Anti- TPO solid phase, enzyme chemiluminescent immunoassay (ECI) where titres above 60 IV/ml were considered significant.
Imaging studies like ultrasonographic examination (USG) of the thyroid gland to determine the morphology and nature of echogenecity was performed in 33 cases. Radioisotope scan with TCM99, was obtained in 14 cases, and fine needle aspiration cytomorphologic (FNAC) study was carried out in 4 cases (grade III goiter in one and nodules on USG in 3 cases).
Student's '1' test was used for statistical analysis where necessary.
Results
Of the 154 infants, children and adolescents referred to the private clinic for thyroid related complaints, 83 had onset of symptoms
above 4 to 5 years of age (Fig. 1). The rest had very early onset congenital hypothyroidism (CH). In 17 of these 83 cases thyroid dysgenesis or dyshormongenesis led to hypothyroidism whereas in 66 cases (80%) based on late onset of symptoms, clinical
findings as mentioned earlier and family data, AITD was suspected (Fig. 1). Based on the initial thyroid antibody status, these 66 cases could be subclassified as thyroid antibody positive in 36 patients (55%) where both the antimicrosomal and anti thyroglobulin antibody or only the former was positive in titers of 1: 100 or higher, and 30 cases where thyroid Ab were lower than 1: 1 00 or negative (Fig.
1). As reported in most studies antithyroid antibody titers tend to be lower in children than in adults and titers of 1: 100 by hemagglutination technique are considered very
significant(2,5,6). Some authors consider antibody titers above 1 in 4 also important(7).
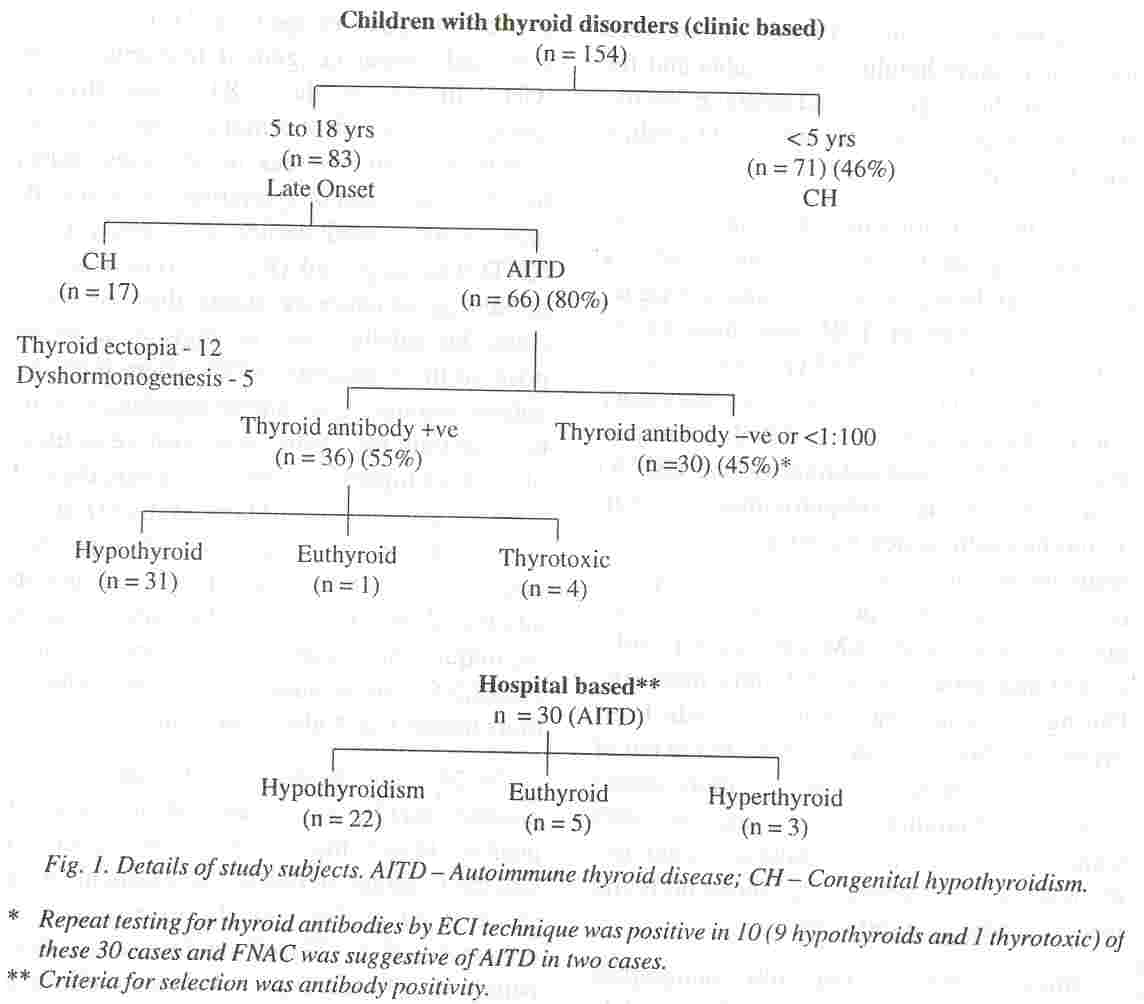
In 24 of the 30 thyroid antibody negative patients AITD was suspected in view of positive family history and other associated clinical findings. In two of these patients with grade II goiter, lymphocytic thyroiditis was demonstrated by FNAC on follow up. One patient developed Type I diabetes mellitus a year .later which has an immunologic basis, and his father developed AITD. In ,10 cases anti microsomal antibodies were demonstrable on repeat testing 6 to 12 months later, using a highly sensitive chemiluminescence assay. Ultrasound scans and TCM99 scans were suggestive of thyroiditis in 4 and 1 patients, respectively. Thus in 12 additional patients more sensitive antibody test or FNAC con- firmed AITD
while 12 others had associated features which were supportive of the
disorder but needed confirmation. Repeat antibody testing or FNAC on further follow up may help in indicating underlying AITD.
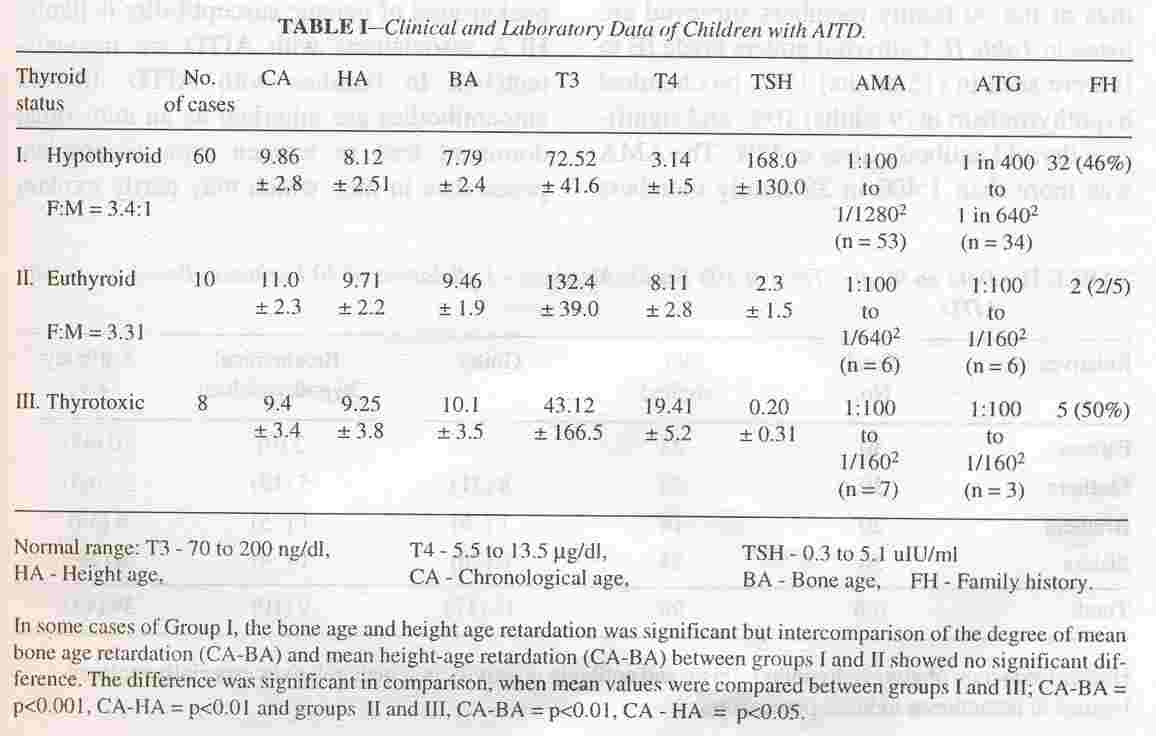
The clinical and laboratory data on 66 children (36 Ab +ve clinic cases and 30 hospital based cases) of AITD with the 12 additional . cases who were later proved to have AITD is shown in Table I.
There was a female predominance with 58 girls and 20 boys, with a
female to male ratio of 2.9: 1. Maximum number of children (40%) were in the 9 to 12 years old age group with 61 % between 6 to 12 years of age. The only child in the series below five years of age was a 3 years old girl with hypothyroidism, a lobulated goiter and higher antibody titers, having 4 adult relatives with AITD.
Based on the functional status of the 2
thyroid gland these 78 cases could be divided into three groups (Table I). Sixty children (77%) had hypothyroidism, 10 cases (13%) were euthyroid and 8 subjects (10%) had thyrotoxicosis. Hypothyroidism predominated, both amongst the antibody positive and negative groups.
In children with hypothyroidism the duration of complaints did not exceed 12 to 18 months. Over 60% presented for goiter, 10% with pain in the thyroid region. Symptoms suggestive of hypothyroidism such as weight gain, growth failure, lassitude, mental apathy, hoarseness, puffiness of eyes, hair loss, poor
appetite and constipation were noted in 54% with varying clinical signs of hypothyroidism. In adolescents there was delay in the onset of puberty with precocious puberty in one girl. Typical myxoedematous appearance was seen in 15% and significant growth failure in 20%. A firm goiter grade IA to II was noted in 74%, with characteristic granular or lobulated surface in more than half of them, a normal sized but soft to firm thyroid gland in 20%; and no palpable thyroid tissue in 6%. Children with euthyroidism presented for goiter (grade IB and III). The duration of symptoms in the 8 children with thyrotoxicosis, 4 girls and 4 boys, ranged between 3 months to one year, the youngest child was 5 years old. The clinical
manifestations were variable and of varying severity but suggestive of thyrotoxicosis with mild proptosis in 6 cases, goiter in all (grade IB to II) with a bruit in 4 cases.
The extent of height age and bone age retardation in relation to chronologie age in the
group of children with hypthyroidism when compared with the euthyroid group was not statistically significant suggesting relatively recent onset, milder dysfunction and/or
gradual progression of hypothyroid state. Subclinical hypothyroidism was noted in 4 of the 60 children with hypothyroidism (group I
-
serum T4 in the lower normal range with minimal elevation of TSH (17 to 35 uIU/ml). Serum T3 values were in the lower normal range in the hypothyroid group in 20%. As stated earlier, 66 (36 clinic cases + 30 hospital cases) of these total of 96 children had anti- body positive thyroid disease (Fig. J) to begin with. The AMA positivity was more common and titres higher, as compared to A TG in 43 (65%) of the 66 AMA positive subjects. Higher titres were observed in children with hypothyroidism. On retesting for thyroid antibodies 6 to 12 months later with more sensitive ECI technique, 10 more Ab negative children with hypothyrodism were
detected to
have significantly elevated thyroid antibody titres and two had AITD on FNAC.
Ultrasonographic studies of the thyroid' gland in 33 children showed poor nonhomogeneous hypoechoic pattern suggestive of thyroiditis in 10 cases. TCM99 scan revealed a patchy uptake suggestive ofthyroiditis in 3 of the 14 instances. FNAC carried out in 4 cases confirmed the diagnosis of chronic lymphocytic thyroditis showing cytomorphologic features of lymphoplasmacytic infiltration with variable degree of hyperplastic follicular epithelium and marked cytoplasmic and peripheral vacuolation.
Family history of thyroid disorders amongst first and second degree relatives was encountered in the clinic cases in 38% (Ab +ve group 44%, Ab -ve group 33%), and in 26% of first degree relatives of the other" group (in 46% of children with hypothyroidism, 56% with thyrotoxicosis and only 2% with euthyroid goiters). The clinical and laboratory find- ings, of the 90 family members' surveyed are listed in Table II. Euthyroid goiters grade IB to II were seen in (IS adults) 17%, biochemical hypothyroidism in (9 adults) 10%, and significant thyroid antibody titres in 43%. The AMA was more than 1 :400 in 39 family members
including 9 with biochemical hypothyroidism and 15 with goiters, but ATG was. elevated in 4, with much lower titres in others. Antibody positivity was maximum in mothers (65%).
Discussion
The expanding spectrum of autoimmunce thyroid disease in children and
adolescents includes three important clinical categories of disorders, acquired hypothyroidism, nontoxic goiter and thyrotoxicosis. The chronic form of thyroiditis (Hashimoto's _or autoimmune thyroiditis) is almost invariably a chronic lymphocytic thyroiditis first reported in childhood in 1938(8), and now believed to be the most common thyroid problem of childhood and adolescence(2,9). The fibrous variant has also been reported in childhood (lO,ll). The most common form of the disease is an asymptomatic, diffuse enlargement of the thyroid gland' described as adolescent goiter(9,12,13).
The primary abnormality in AITD is un- known. Environmental factors operating on a background of genetic susceptibility is likely. HLA associations with AITD are inconsistent(l4). In families with AITD, thyroid autoantibodies are inherited as an autosomal dominant trait in women with incomplete penetrance in men which may partly explain
the marked predominance in females(14,15). Increased incidence in
presence of chromosomal abnormalities such as Down's or Turner's syndrome or association with other endocrinopathies as in Type I autoimmune diabetes mellitus (30%) is known(15,16). Epstein Barr and retroviruses and Yersinia
infections lead to antithyroid antibody formation by molecular rnimicry(3,7). High iodine intake possibly due to increased immunogenecity of thyroglobulin
or direct toxic effect on thyroid tissue may lead to AITD(17). An
inherited defect in immune surveillance with partial defect in T
suppressor cell activity leading to defective suppression of thyroid
directed T helper cells causing increased antibody production is postulated(18,19). The antibodies are TSH like leading to hyperplasia and excess activity of the thyroid gland in Grave's disease and cytotoxic damage leading to hypothyroid- ism in Hashimoto' s(18, 19).
AITD involves 3-4% of general population while in elderly females it is estimated to be as high as 16%(19). The incidence in children is not
known though a true increase may be due to greater clinical awareness better diagnostic techniques or other factors. The incidence is quoted as 5 new cases per 1000 subjects per year with a prevalence of 1.3% in school children 11 to 18 years 0Id(15,20). Both in Japan and in USA the incidence in adolescent girls is 0.8% and 1.6%(15). In our survey of more
than 1800 school children (age 8 to 16 years) for iodine deficiency ,significantly elevated thyroid antibodies. were detected in 4% of 224 children having goiters and in low titers, 0.97% of normal control group (unpublished data).
AITD generally involves children above the age of 4 to 5 years and is a leading cause of juvenile hypothyroidism after the age of six years(2,15). Though rare below the age of 3 years, it is recently reported in infancy(6,11).
The mean age at presentation in this series was 10.1 :t 2.9 years, similar to other reported series, as 10.25 and lOA years(21,22). Female preponderance which is as high as 9.5:1 in adults is not seen in prepubertal girls where it is only twice as common(2,7,15). In our study the female to male ratio was 2.9.
These children present with euthyroid goiter, toxic thyroiditis or hypothyroidism; the clinical behavior being heterogenous and un- predictable. The incidence of euthyroid goiters has ranged from 7.5% to 70% and that of hypothyroidism from 21 % to 85%(21,22). A
large majority of chronic lymphocytic thyroidits present for asymptomatic
diffuse goiter with a firm symmetrical thyroid enlargement in 80%, often having a finely granular or bosselated
surface(12). In those presenting with marked growth failure, delayed
bone age and other features of hypothyroidism, the thyroid gland is
often not palpable due to the aggressive fibrous variant of
AITD(2,23). In the present series amongst those with hypothyroidism over 60% presented for goiter and in 6% thyroid tissue was not palpable. Euthyroid goiters were less frequently seen (13%). In some euthyroid
children hypothyroidism may develop subsequently. Occasionally, features of transient hyperthyroidism are seen during the early phase of the disease as was also observed in two cases in this series, before hypothyroidism finally supervened. Thyrotoxicosis due to Grave's disease involved 10% of the present series, similar to 7.5% and 9% incidence reported by others(21,22). Symptoms of hyperthyroidism have been described in absence of any demonstrable elevation in thyroid hormones in children with chronic lymphocytic thyroiditis(12,21,22).
Diagnostic evaluation involves assessment of thyroid function and testing for thyroid autoantibodies. Imaging studies, ultrasonographic and thyroid scanning with Technetium pertechnetate (TCM99) though not confirmatory, are additional helpful diagnostic parameters. Nonhomogeneous
patchy echogenecity or patchy uptake was noted in 30% and 21 %, respectively of the patients tested. FNAC is not recommended as a routine procedure and is not essential for diagnosis of Aim in children(2,7,12,13). It may be undertaken in presence of nodules (especially solitary) or a large thyroid gland nonresponsive to L-thyroxine therapy or in presence of regional lymphadenopathy.
A variety of antibodies have been identified in AIm, but the thyroglobulin antibody and thyroperoxidase (microsomal) antibody tests are usually done. These antibodies are not directly cytotoxic but reflect the presence of damage to thyroid epithelium and lymphocytic infiltration. The frequency of antibody positivity is lower, and in lower titres in children, as compared to adults, and thyroid autoantibodies may be absent or fluctliate in juvenile AIID(5,12,13,24-26). Low titres may be noted in other thyroid diseases as well and in end stage chronic lymphocytic thyroiditis with hypothyroidism. RIA, ELISA and other recent techniques like chemiluminescence immuno assay are more sensitive for antibody detection. Thyroid antibody positivity increased to almost 85% when more than one test was used(25,26). About 2% of classic goitrous
thyroiditis and 30% of the atrophic type
have
no
detectable antibodies( I 9). In the present study only 55% of the clinic group tested antibody positive initially, with higher positivity for AMA, but on repeat testing with .ELISA and! or chemiluminescence 6 to 12 months later, ten more cases tested very significantly positive.
The autoimmune thyroid disease have a definite familial aggregation. Thyroid anti- bodies can be demonstrated in the first degree relatives of patients with thyroiditis and in
families of patients with hyperthyroidism(23,27). The ability to form antibodies is under polygenic control and is of moderate heritability with upper limit of about 50%(27). Juvenile AIT has an impressive familial association with a remarkable dose effect of parental autoimmunity(15). Excluding the proband, the prevalence of thyroid autoantibodies in the siblings was 71 %, 54% or 29% if both parents, one parent or neither parent respectively had thyroid antiobodies(28). Very few studies on the family members of children with AIm are available. One of the earlier studies indicated antibody positivity in the family members above 50%(29). The prevalence of auto- immune thyroid disease in this series in first and second degree relatives as elicited from history was 44%, with antibody positivity
in 43% of first degree relatives; 65% of mothers and nearly 30% of
siblings. There is a higher risk for developing hypothyroidism in
antibody
positive subjects(l5,19,29). Unlike our experience with hypothyroidism due to dyshormonogenesis where the hereditary transmission is autosomal recessive with sib- ling involvement and no disease in the parents, in AIm parents, mothers were particularly affected.
Some studies suggest that AIm is a self limited disease and thyroid hypofunction
recovers spontaneously(9,26). Thyroid function needs to be
reassessed after stopping or tapering therapy for a period of six to eight weeks after initial treatment for 2 or 3 years. Hypo or hyperfunction of the thyroid gland needs adequate replacement (Ievothyroxine) or suppressive (antithyroid drugs) treatment with effective monitoring. Corticosteroids or immunosuppressives are not indicated. Thyrotoxicosis associated with chronic lymphocytic thyroiditis runs a milder course. There are no good predictors for the outcome but female sex and well established
hypothyroid state at the time of diagnosis probably indicate a need for life
long treatment. The management of euthyroid goiters with thyroiditis
has been a matter of discussion. This group needs frequent monitoring. Large euthyroid goiters are often treated with suppressive levothyroxine therapy as untreated chronic lymphocytic thyroiditis may lead to multinodularity with progressive hypofunction(7,12).
Autoimune thyroiditis is the most important cause of acquired hypothyroidism with significant familial and genetic implications. As noted in our study diagnosis of AIm in children may lead to the detection of disease in adult family members, particularly mothers who may become beneficiaries of early intervention.
Acknowledgements
We wish to thank Sir H.N. Medical Research Society (HNMRS) for supporting part of the study and The Bai Jerbai Wadi a Hospital for Children and Institute of Child Health Research Society. We also wish to thank all the patients and their families for
,
their kind co-operation and the laboratory staff
of both the institutions and secretarial staff of HNMRS.
|
1.
LaFranchi S. Thyroiditis and acquired hypothyroidism. Pediatr Ann 1992; 21: 29-35.
2. Foley TP Jr. Disorders of the thyroid in children. ln: (ed) Pediatrics Endocrinology, 1st edn. Philadelphia, W.B. Saunders Co., 1996; pp 173-177.
3.
Fisher DA, Pandian MR, Carlton E. Autoimmune thyroid disease: An expanding spectrum. Pediatr Clin N Am 1987; 34: 907-918.
4.
Desai MP, Colaco MP, Samuel AM, Vas FE. Etiology of childhood hypothyroidism. Indian Pediatr 1989; 26: 212-222.
5.
Hahn HB, Hayles AB, Woolner LB. Lymphocytic thyroiditis in children. J Pediatr 1965; 66: 73-78.
6.
Foley PT, Abbassi V, Copeland KC, Draznin MB. Hypothyroidism caused by chronic autoimmune thyroiditis in very young infants. N Eng J Med 1994; 330: 466-468.
7.
Dallas JS, Foley TP Jr. Hypothyroidism. In: Pediatric Endocrinology: A Clinical Grade, 3rd edn. Ed. Lifshitz F Newe York, Marcel Dekker, 1995; pp 391-399.
8.
Hellwing CA. Lymphadenoid goiter. Arch Patho11938; 35: 838-841.
9.
Rallison M, Dobyns BM, Keating FR, Rau JE, Tyler FH. Chronic thyroiditis: Occurrence and natural history of chronic lymphocytic thyroiditis in children. J Pediatr 1975; 86: 675- 682.
10.
Foley TP Jr, Schubert WK, Marnall RT. Chronic lymphocytic thyroiditis and juvenile myxedema
in uniovular twins. J Pediatr 1968; 72: 201-207.
11.
Ostergaard GZ, Jacobsen BB. Atrophic, autoimmune thyroiditis ininfancy: A case report. Horm Res 1989; 31: 190-192.
12.
Hung W, Chandra R, August GP, Altman PRo Clinical, laboratory and histologic observations in euthyroid children and adolescents with goi- ter. J Pediatr 1973; 82: 10-16.
13.
Monteleane JA, Danis RK, Tong SK, Ramos CV, Peden VH. Differentation of chronic lymphocytic thyroiditis and simple goiter in pediatrics. J Pediatr 1973; 83: 381-385.
14.
Weetman AP, McGregor M.Autoimmune myroid disease: Developments in our understanding. Endocrine Rev 1984; 5: 309-312.
15.
McGregor AM, Hall R: Thyroiditis. In: Endocrinology, Vol I, 2nd edn. Eds, Degroot LJ, Besser GM, Cahill GF, Marshall JC, Nelson DH, Odell WD, et at. Philadelphia, W.B. Saunders Co 1989; pp 683-70 I.
16.
Gray RS, Borsey DQ, Seth J. Prevalence of subclinical thyroid failure in insulin dependent diabetes. J Clin Endocrinol Metab 1980; 50: 1034-37.
17.
Safran M, Paul TL, Rop E, Braverman LE. Environmental factors affecting autoimmune thy- roid disease Endocrinol Metab Clin N Am 1987; 16: 327-342.
18.
Volpe R. Autoimmunity causing thyroid dysfunction. Endocrinol Metab Clin N Am 1991; 20: 565-586.
19.
Volpe R Autoimmune thyroiditis. ln: The Thyroid, 6th edn. Eds. Braverman LE, Utiger RD, Philadelphia, J.8. Lippincott,
1991; pp 921-941.
20.
Rallison MC, Dobyns BM, Merkle W, Bishop M, Lyon JL, Stevens W. National history of thyroid abnormalities, prevalence, incidence and regression of thyroid disease in adolescents and young adults. Am J Med 1991; 91: 63- 70.
21.
Gruneiro L, Torcansky S, Rivavola MA, Bergada C. Variations in clinical, hormonal, serological expressions of chronic lymphocytic thyroiditis in children and adolescents. Clin Endocrinol 1982; 16: 19-28.
22.
Saxena KM, Crawford JD. Juvenile lymphocytic thyroiditis. Pediatrics 1962; 30: 917-926.
23. Hall R, Owen SG, Smart CA. Evidence for a
genetic predisposition to formation of thyroid antibodies. Lancet 1960; 2: 187-188.
24.
Loeb PO, Drash AL. Kenny FM. Prevalence of low titre and negative anti thyroglobulin anti- bodies in biopsy proven juvenile lymphocytic thyroiditis. J Pediatr 1973; 82: 17-21.
25.
Hopwood NJ, Rabin BS, Foley TP, Peake RL.Thyroid antibodies in children and adolescents with thyroid disorders. J Pediatr 1978; 93: 57 -61.
26.
Maenpaa J, Raatikka M, Rasanen J, Taskinen E, Wager O. Natural course of juvenile autoimmune thyroiditis. J Pediatr 1985; 107: 898-904.
27. Hall R, Dingle PR, Roberts OF. Thyroid anti- bodies: A study of first degree relatives. Clin Genet 1972; 3: 319-321.
28.
Burek CL, Hoffman WH, Rose NR. The presence of thyroid antibodies in children and
adolescents with autoimmune thyroid disease and their parents. Clin Immunol Immunopathol 1982; 25: 395-399.
29.
Doniach 0, Nilsson LR, Roitt 1M. Autoimmune thyroiditis in children and adolescents II. Immunological correlations and parent study. Acta Pediatr Scand 1965;
54: 260-266.
|
