Atrial standstill or paralysis has been defined as
the absence of any electrical or mechanical activity of atria [1-3]. In
atrial standstill, there is no evidence of atrial electrical activity in
the surface ECG leads or on electrophysiological evaluation. We report
atrial standstill in acute myocarditis that persisted even after the
resolution of myocarditis.
Case Report
A 10-year-old girl presented with congestive heart
failure (CHF) following an episode of low grade fever. She had no family
history of cardiac illness, pacemaker implantation, embolic events or
skeletal muscle disease. Investigations revealed a normal total
leukocyte count (7x 10
9/L)
with 18% neutrophils. Serum electrolytes were in the normal range. The
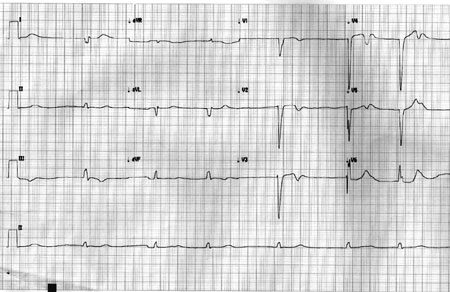
ECG at the time of presentation showed absent P waves and a wide QRS
regular escape rhythm at 40/min (Fig. 1). Cardiac troponin
T was raised (1793 ng/L). Echocardiogram showed hypokinesia of the left
ventricle and mild left ventricular (LV) dysfunction. Her Anti-ds DNA
and anti-nuclear antibody titres were normal. She had received
intravenous immunoglobulins, ampicillin and ranitidine before she was
referred to our unit. We continued conservative management with bed
rest, diuretics, and digoxin. Her CHF improved, and subsequently she was
discharged. At review visit 6 weeks later, she had no CHF but had the
same ventricular escape rhythm and exertional fatigue. Echocardiogram
showed improved LV function (ejection fraction 51%). Mitral and
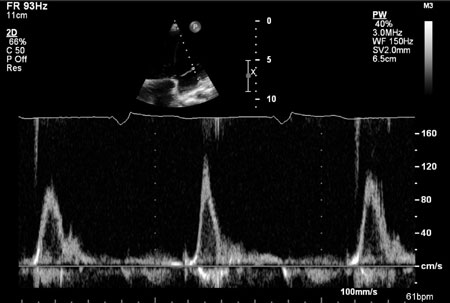
tricuspid inflow Doppler study showed absence of "a" wave suggestive of
total atrial standstill (Fig. 2). An evaluation done for
neuromuscular disease showed normal muscle strength and normal
electromyography.
 |
|
Fig. 1 The 12 Lead ECG showing
idioventricular rhythm with absence of P wave and wide QRS
escape rhythm.
|
 |
|
Fig. 2 Mitral inflow pulse wave
Doppler showing absence of ‘a’ wave and paced rhythm at
60/minute.
|
Electrophysiology study (EPS) showed no evidence of
atrial electrical activity. There was no atrial capture even at maximum
pacing output (25mA), from multiple atrial sites (Web Fig.
I), consistent with atrial standstill. She underwent a pacemaker
implantation, and subsequently the right ventricular pacing parameters
were normal. Her ECG after 10 months showed no p waves, and the
fluoroscopy and echocardiography showed no suggestion of atrial
contraction. Interrogation of the pacemaker confirmed that she was
totally pacemaker-dependent.
Discussion
Atrial standstill has been reported to occur in
inherited myopathies, valvular cardiomyopathies, digitalis or quinidine
intoxication, hypoxia, hyperkalemia, myocardial infarction, systemic
lupus erythematous, and Chaga’s disease. Atrial standstill secondary to
myocarditis, persisting long after the acute phase, is extremely rare.
Atrial standstill could be defined as partial or
total [4]. In partial atrial standstill, conduction disturbance within
the right atrium alone is a more common finding. The absence of P waves
in surface ECG occur in sinus node arrest with junctional escape rhythm
which is thus a differential diagnosis of atrial standstill. However, in
sinus arrest, the atria can be shown to be excitable by pacing whereas
in atrial standstill, atria are non-excitable and cannot be captured
even with high pacing outputs. This results in lack of mechanical
functioning of atria, and predispose to intra-atrial thrombus formation.
Our patient had a wide QRS escape rhythm with no P
waves. This indicates an escape rhythm from below the level of His
bundle (Infra-Hisian). The inability to capture the atria even at high
current output during the EPS confirmed atrial standstill in our case.
We used digoxin for LV dysfunction in spite of patient having
bradycardia because digoxin has minimal action on infra-Hisian tissues,
and is less likely to suppress the escape rhythm. However, a stand by
temporary pacemaker was kept ready, and patient was closely monitored.
Isoprenaline, which can be used to increase the heart rate, can also
worsen the conduction in infra-Hisian disease, and hence was not used.
She was closely monitored in the acute phase, and as there was a
possibility that her rhythm may normalize once her myocarditis resolves,
we waited for six weeks before implanting a permanent pacemaker.
Persistent atrial standstill is a rare disorder [5],
and that occurring after myocarditis is even rarer. Straumanis, et al.
[6] have reported a 11-year-old child with biopsy-proven necrotizing
acute myocarditis causing atrial standstill. However, this was transient
and resolved after 3 days of treatment with methylprednisolone. Our
patient had persistence of atrial standstill, even after the ventricular
dysfunction recovered. Abdelwahab, et al. [7] have reported a
case of multiple atrial arrhythmias (atrioventricular node re-entry and
two different focal atrial tachycardias) originating from the remaining
atrial myocardium after global scarring of both atria following a remote
viral myocarditis. Talwar, et al. [8] reported a case series in
which two of the patients had lymphocytic infiltrates on right
ventricular endomyocardial biopsy. In another case series of 11 patients
with atrial standstill reported by Nakazato, et al. [9], three
had histological evidence of chronic myocarditis. None of their patients
had a presentation suggestive of acute myocarditis.
Pathological involvement of atria in atrial
standstill can be localised or diffuse. Our patient likely had a total
atrial standstill as evidenced in mitral pulse wave Doppler showing
absence of ‘a’ wave. As atrial standstill is a well-known cause of
cardiogenic embolism [9], anti-coagulation is mandatory; our patient was
started on Warfarin. The management of patients with atrial standstill
include anticoagulation in all patients, and pacemaker implantation in
patients who have symptomatic bradycardia due to insufficient rates of
escape rhythm.
We conclude that acute myocarditis can rarely cause
extensive damage to the atria causing loss of electrical and mechanical
function. This may herald atrial standstill, which can persist even
beyond the acute phase when other changes have recovered.
Contributors: MAP and SP: case management,
drafting of manuscript; AT and NN: case management critical revision of
manuscript. All authors approved the final version.
Funding: None; Competing interests: None
stated.
References
1. Surawicz B. Electrolytes and the
electrocardiogram. Am J Cardiol. 1963;12:656.
2. James TN. Myocardial infarction and atrial
arrhythmias. Circulation. 1961; 24:761.
3. James TN, Rupe CE, Monte R. Pathology of the
cardiac conduction system in systemic lupus erythematosus. Ann Intern
Med. 1965;63:402.
4. Lévy S, Pouget B, Bemurat M, Lacaze JC, Clementy
J, Bricaud H. Partial atrial electrical standstill: report of three
cases and review of clinical and electrophysiological features. Eur
Heart J. 1980;1:107-16.
5. Allensworth DC, Rice CI, Lowe G. Persistent atrial
standstill in a family with myocardial disease. Med Am J.
1969;47:775-84.
6. Straumanis, John PW, Henry BC, Christopher LR.
Resolution of atrial standstill in a child with myocarditis. PACE.
1993;16:2196-202.
7. Amir A, John LS, Ratika P, Magdy B, Martin G.
Mapping and ablation of multiple atrial arrhythmias in a patient with
persistent atrial standstill after remote viral myocarditis. PACE.
2009;32:275-7.
8. Talwar KK, Dev V, Chopra P, Dave TH, Radhakrishnan
S. Persistent atrial standstill — clinical, electrophysiological and
morphological study. PACE.1991;14:1274-81.
9. Yuji N, Yasuro N, Teruhikoa H, Masataka S,
Shunsuke O, Hiroshi Y. Clinical and electrophysiological characteristics
of atrial standstill. PACE. 1995;18:1244-54.

